Apr 17 2017
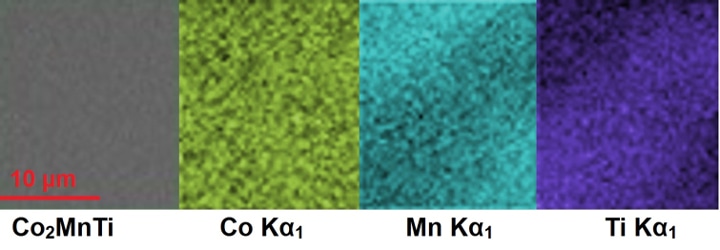 A microscopic look at the atomic structure of a cobalt-manganese-titanium mixture (Co2MnTi) that is one of the newly predicted and manufactured magnetic materials. Each color shows the distribution of a different element. The uniformity for each material matches the predictions for a stable three-element material. Credit: PELIN TOZMAN, AMBER AND CRANN INSTITUTE, TRINITY COLLEGE, DUBLIN, IRELAND
A microscopic look at the atomic structure of a cobalt-manganese-titanium mixture (Co2MnTi) that is one of the newly predicted and manufactured magnetic materials. Each color shows the distribution of a different element. The uniformity for each material matches the predictions for a stable three-element material. Credit: PELIN TOZMAN, AMBER AND CRANN INSTITUTE, TRINITY COLLEGE, DUBLIN, IRELAND
Two innovative magnetic materials have been proposed and developed, atom-by-atom, by material scientists by means of high-throughput computational models. The achievement could lead to the start of a new age in the extensive modeling of innovative magnetic materials at unprecedented pace.
In spite of the fact that magnets are abundantly used in the day-to-day life, in reality, they are very rare. Of all the known inorganic compounds, just 5% display traces of magnetism. Among these compounds, only very few can be used in real-world applications since their properties, such as, magnetic permanence and effective temperature range, vary.
As these materials are comparatively scarce, they are difficult to obtain or expensive, forcing researchers to look for new alternatives as magnets are highly significant in applications that range from motors to magnetic resonance imaging (MRI) machines. The conventional process includes not just trial and error analysis because scientists develop disparate molecular structures for discovering a material that has magnetic properties. However, numerous high-performance magnets are peculiar in chemical and physical trends eluding intuition.
In a recent research by materials scientists from Duke University, a shortcut to this process has been developed. The scientists demonstrate the potential to estimate magnetism in new materials by using computer models with the ability to screen innumerable candidates in short order. Furthermore, to prove the validity of the process, they have developed two magnetic materials not known earlier.
The outcomes of the research have been reported in the April 14, 2017, issue of the journal Science Advances.
Predicting magnets is a heck of a job and their discovery is very rare. Even with our screening process, it took years of work to synthesize our predictions. We hope others will use this approach to create magnets for use in a wide range of applications.
Professor Stefano Curtarolo, Director, Center for Materials Genomics, Duke University
The researchers concentrated on a class of materials known as Heusler alloys, that is, materials formed of atoms of three different elements arrayed in one among three discrete structures. Taking into account the entire probable arrangements and combinations available by using 55 elements, the research team could select from a total of 236,115 prospective prototypes.
The research team developed each prototype atom-by-atom by means of a computational model to arrive at the prospective prototypes. When the manner in which the atoms would possibly interact and the energy necessitated by each structure were computed, the list was narrowed down to 35,602 potentially stable compounds.
From this point, the research team carried out a highly strict investigation of stability. In general, materials get stabilized into the arrangement that necessitates the minimum energy to maintain. Each compound was examined against other atomic arrangements, and those compounds that were beaten by the competitive materials were rejected, which narrowed the list to 248 compounds.
Only 22 of the 248 compound exhibited a calculated magnetic moment. The final step involved rejecting materials with competing alternative structures that were very close for comfort. This left the research team with 14 compounds to develop from a theoretical model into real-world compounds.
However, as is normal in a laboratory, developing new materials is easy to conceptualize but hard to perform.
It can take years to realize a way to create a new material in a lab. There can be all types of constraints or special conditions that are required for a material to stabilize. But choosing from 14 is a lot better than 200,000.
Corey Oses, Doctoral Student, Duke University
In order to synthesize the material, Curtarolo and Oses sought the assistance of Stefano Sanvito, professor of physics at Trinity College in Dublin, Ireland. Sanvito spent many years trying to develop four such materials, and was successful in developing two.
As predicted, both were magnetic materials.
The first newly invented magnetic material was formed of cobalt, magnesium and titanium - Co2MnTi. The researchers accurately predicted the new magnet’s characteristics by comparing the measured characteristics of magnets having similar structures. Specifically, the temperature at which the new material lost its magnetism was estimated to be 940 K, or 1232 °F. During the analysis, the real “Curie temperature” was found to be 938 K, or 1228 °F, which is an unusually high temperature. This fact, together with the fact that the material lacks rare earth elements, renders it potentially useful in numerous commercial applications.
“Many high-performance permanent magnets contain rare earth elements,” stated Oses. “And rare earth materials can be expensive and difficult to acquire, particularly those that can only be found in Africa and China. The search for magnets free of rare-earth materials is critical, especially as the world seems to be shying away from globalization.”
The other material was formed of manganese, platinum and palladium (Mn2PtPd). This material was found to be an antiferromagnet, that is, the electrons in the material are evenly divided in their arrangements. This causes the material to lack its own internal magnetic moment; however, it renders the electrons to respond to external magnetic fields.
Although this characteristic does not find many applications other than hard drives, magnetic field sensing and random access memory (RAM), magnets of such kind are highly hard to predict. Yet, the computations of the researchers for its different characteristics were accurate.
It doesn’t really matter if either of these new magnets proves useful in the future. The ability to rapidly predict their existence is a major coup and will be invaluable to materials scientists moving forward.
Professor Stefano Curtarolo, Director, Center for Materials Genomics, Duke University
The Science Foundation of Ireland, the EU Commission and the National Science Foundation (DGF1106401) supported this study.