Nov 9 2017
A highly robust gel made up of huge amounts of ionic liquid has been developed by researchers. The research team was headed by Professor MATSUYAMA Hideto and Assistant Professor KAMIO Eiji (Kobe University Graduate School of Science, Center for Membrane and Film Technology). These findings have been published in the November 8 edition of Advanced Materials.
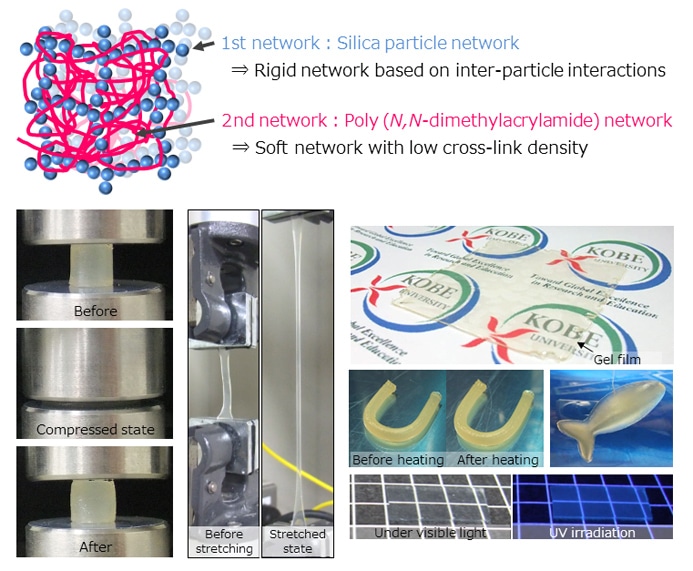 Diagram of the inorganic/organic double network within the strong ion gel, demonstrations of its resilience (compression test, stretching test) and moldability (film, tube, fish shape) CREDIT: Kobe University
Diagram of the inorganic/organic double network within the strong ion gel, demonstrations of its resilience (compression test, stretching test) and moldability (film, tube, fish shape) CREDIT: Kobe University
Ionic liquid is considered to be a substance produced exclusively from ions, which has distinctive properties – for instance, it does not evaporate at normal pressures or temperatures, and it has great thermal stability. Gels that comprise of ionic liquid are called ion gels. With the same properties as ionic liquids and their potential to retain liquid form, they could be employed as membranes for gas separation and as electrolytes for rechargeable batteries. However, practical applications are limited due to the low mechanical strength of typical ion gels.
The research team developed a double network within ionic liquid, merging a network of inorganic silica particles with a network of organic polymers. This strongly enhanced the resilience of the ion gel, and the gel that they produced is capable of withstanding more than 25 MPa of compressive strength without breaking. The strength of the recently-developed robust ion gel comes from the special interpenetrating double network. The brittle silica particle network breaks and then dissipates the loaded energy when stress is applied to the gel. The network is allowed to self-recover by the physical interaction between the silica particles. Most of the ionic liquid present in the gel does not vaporize, hence it can be stored for a long time in a stable condition. Even exposing it to a high temperature vacuum does cause any damage to its performance, thus it can also be employed in high temperature fields.
The gel attained from this research could be employed in CO2 separation membranes or used as electrolytes for rechargeable batteries. The research team will work together with businesses in order to discover practical applications for this gel. They will also continue to examine the strengthening mechanism in a much detailed manner, and target for a stronger, high-performance gel by designing the perfect network.