Nov 17 2017
Germanium, considered to be a more efficient elemental semiconductor than silicon, was the material of choice in the early history of electronic devices. Eventually, the high cost of developing germanium crystals concealed its efficiency, and silicon thus captured the field.
However, this supremacy is challenged by new research establishing an economical method for growing crystalline thin-film germanium by using a process called as van der Waals epitaxy.
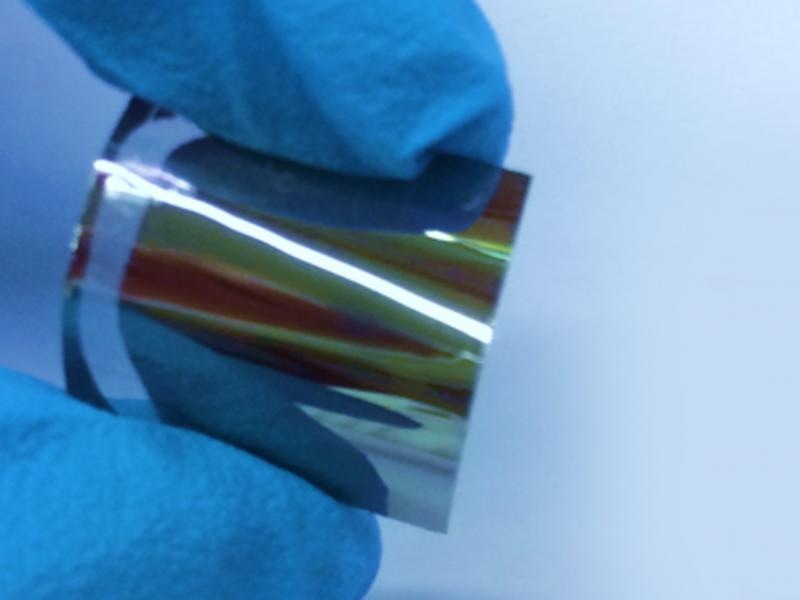 Credit: Rensselaer Polytechnic Institute
Credit: Rensselaer Polytechnic Institute
“This is the first time strain-free van der Waals epitaxy of an elemental semiconductor has been demonstrated,” said Aaron Littlejohn, a doctoral candidate in the laboratory of Gwo-Ching Wang, professor of physics, applied physics, and astronomy at Rensselaer Polytechnic Institute. “Our research found a narrow window, a very specific set of conditions that work.”
The technique interestingly produces flexible germanium, which can be removed from the substrate.
Our germanium film could be used as a thin-film nanomembrane, which could be integrated into electronic devices more easily than nanocrystals or nanowires. It could also serve as the substrate for the subsequent deposition of additional materials for flexible transistors and solar cells, or even wearable optoelectronics.
Aaron Littlejohn, First Author
The research was published in this week’s edition of The Journal of Applied Physics, from AIP Publishing.
The atoms in semiconducting materials must be steadily arranged in a precise crystal lattice in order to efficiently conduct electrical charge. To produce that consistent lattice, semiconductor fabricators make use of a phenomenon called epitaxy, and deposit a thin film layer of the selected semiconducting material on an expendable crystalline substrate. As they are deposited, the atoms of the semiconducting material and the film have the same pattern, the perfectly matched layers develop strong chemical bonds for optimal electrical charge carrier mobility.
If the periodicities, or atomic spacings, of the substrate and the semiconductor are not a good match, conventional epitaxy produces a material compromised by defects and strain, which decreases carrier mobility. There are few good matches for germanium, and those that exist, such as gallium arsenide, are expensive, driving up the cost of manufacturing.
The researchers solved the problem by growing a germanium film on a substrate of muscovite mica, a material whose atoms align in layers, with no chemical bonds between layers. The surface of a layer of mica is free of dangling bonds, guaranteeing that germanium, as it is deposited, will not chemically bond with or even adhere to the periodicity of the mica.
The arrangement of germanium atoms is instead guided by van der Waals forces, weakly attractive interactions that happed between neutral atoms, based on the probabilistic nature of electrons. This permits the germanium to grow in a relaxed film, in spite of the dramatically different crystal structures of the two materials (a 23% difference in atomic spacing).
The researchers grew germanium films about 80 nm thick on muscovite mica substrates 75 mm long, 25 mm wide and 0.26 mm thick. By changing the substrate temperature during deposition and annealing in the range of 300-500 oC, the researchers discovered that the crystal lattice stabilizes at about 425 oC.
Previous research implies that elemental semiconductors cannot be epitaxially grown on mica using van der Waals forces at any elevated temperature, but we have now shown otherwise. With the success of our germanium film grown on mica at a practical temperature, we anticipate that other nonlayered elemental or alloyed materials can be grown on mica via van der Waals epitaxy.
Aaron Littlejohn
Littlejohn and Wang were accompanied on the research by Yu Xiang, Elma Rauch, and Toh-Ming Lu. "van der Waals epitaxy of Ge films on mica,” can be found using DOI: 10.1063/1.5000502.
The research accomplishes The New Polytechnic, a growing paradigm for higher education which recognizes that international challenges and opportunities are so immense such that they cannot be adequately addressed by even the most talented person working all alone.
Rensselaer aids as a crossroads for collaboration — working along with partners across sectors, disciplines and geographic regions — in order to address complex universal challenges, using the most advanced technologies and tools, many of which are developed at Rensselaer. Research at Rensselaer focuses on addressing some of the world’s most persistent technological challenges — from sustainable development and energy security to human health and biotechnology. The New Polytechnic is considered to be transformative in the global impact of research, in its innovative pedagogy, and also in the lives of students at Rensselaer.