Jan 3 2018
The industrial production of hydrogen and other practical compounds from natural gas involves the use of transition-metal catalysts such as cobalt and nickel. Scientists accomplish this conversion by performing steam reforming, a method in which methane is heated by using steam in the presence of catalyst, thereby generating carbon monoxide and hydrogen.
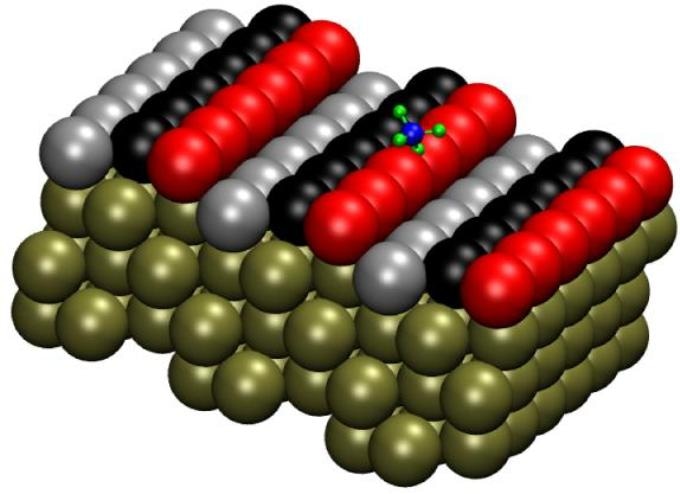 The Pt(211) surface has three-atom-wide terraces and one-atom-high steps. Researchers labeled the rows of atoms as follows: atoms on the step edge as "step" (red), the middle row as "terrace" (black) and the final row as "corner" (gray). Image credit: Han Guo.
The Pt(211) surface has three-atom-wide terraces and one-atom-high steps. Researchers labeled the rows of atoms as follows: atoms on the step edge as "step" (red), the middle row as "terrace" (black) and the final row as "corner" (gray). Image credit: Han Guo.
Transition metals have exceptional catalytic potential and it is known to scientists that the most important reactions take place at the surface of the catalysts. To date, the hunt for more active catalysts has been mainly dependent on trial and error, and also on the presumption that catalyzed reactions occur on step edges as well as other atomic defect sites in metal crystals.
An international team of scientists from Switzerland, the Netherlands and the United States has integrated various experiments by applying state-of-the-art infrared methods with quantum theory to investigate methane dissociation reactions in depth. This is the first time that the exact place of occurrence of the most important reactions on the catalyst’s surface has been demonstrated. Although the team mainly used platinum (Pt) as the catalyst to disintegrate methane, the model can be used for other transition-metal catalysts, for example, nickel. The outcomes of the study have been reported in The Journal of Chemical Physics, published by AIP Publishing.
“A tested predictive theory with chemical accuracy could change the way one searches for new catalysts and make the search more efficient and cheaper,” stated Rainer Beck, Professor of chemical science and engineering at cole Polytechnique Fdrale de Lausanne (EPFL), who is co-author of the paper.
At the atomic level, a platinum catalyst’s surface, and also the surface of other metal crystals, might comprise terraces, steps and other flaws that are observed to be significant “sites” in the catalytic method.
The researchers made use of infrared laser pumping to stimulate the methane molecules, in order to attain chosen vibrational and rotational quantum states. They then adopted reflection-absorption infrared spectroscopy (RAIRS), a nonintrusive method, to observe methane disintegration on different sites in the Pt(211) crystal. RAIRS enables real-time monitoring of chemical reactions at the time of methane deposition on the Pt surface by making note of site-specific uptake curves for chemisorbed methyl species on terraces sites and steps. Using these measurements, the team can then ascertain methane’s reactivity levels on each site.
The team also adopted the Reaction Path Hamiltonian model, a quantum theory framework, to compute the potential energy surface and investigate the dynamics of the chemical reactions. The outcomes of the study indicated that dissociation reactions are, at the minimum, two orders of magnitude more effective on the steps when compared to the terraces. In addition, there was no reaction recorded on another type of site on the surface, known as “corner atoms,” which was positioned between the steps and the terrace.
We demonstrated that it is possible to use RAIRS detection for state- and surface-site specific measurements of methane reactivity and to compare the effect of vibrational excitation on reactivity on the steps and terraces of a catalyst surface. This new area of study provides another level of detail in detecting methane’s dissociation products.
Rainer Beck, Professor of chemical science and engineering at cole Polytechnique Fdrale de Lausanne (EPFL)