Jan 11 2018
Molecular machines, a lot smaller than single cells, may soon be able to deliver drugs to destroy cancer cells or patrol the body for signs of disease. But a number of applications of these machines require big arrays of rock-hard moving parts, which would be hard to build with usual biological structures.
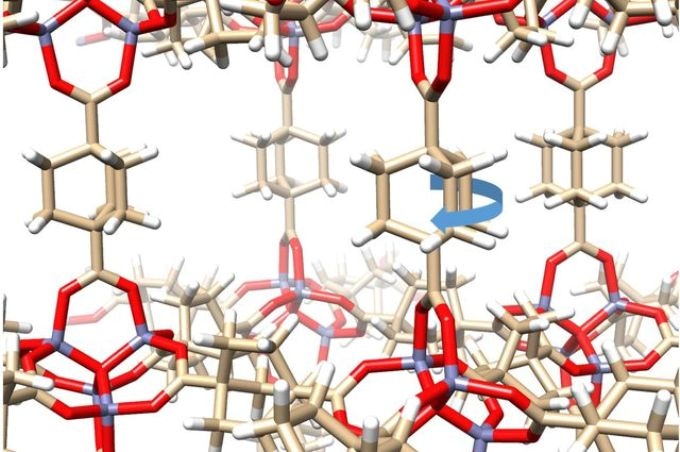 UCLA researchers formed a crystal out of molecules that each has a solid exterior but contains moving parts. (Image credit: Kendall Houk Laboratory/UCLA)
UCLA researchers formed a crystal out of molecules that each has a solid exterior but contains moving parts. (Image credit: Kendall Houk Laboratory/UCLA)
Molecules that make up the solid crystals found in nature are normally so tightly packed together that there is no room for any of them to move. Therefore, regardless of their durability and strength, solid crystals have largely not been considered for use in molecular machines, which must have moving parts that can react to stimuli.
Currently, UCLA researchers have developed a crystal out of molecules that look like gyroscopes with solid frames. Since each molecule has an exterior case surrounding a rotating axis, the crystal has a solid exterior but possesses moving parts.
The new crystal, explained in the journal Proceedings of the National Academy of Sciences, is the first proof that a single material can be both moving and static - or amphidynamic.
For the first time, we have a crystalline solid with elements that can move as fast inside the crystal as they would in outer space.
Miguel García-Garibay, Senior Author
To develop repetitive arrays of molecular machines, or smart materials, researchers have repeatedly turned to liquid crystals, which are designed for use in LCD TV screens but also are found in nature. However, liquid crystals are comparatively slow: each molecule must completely change orientation to change how it interacts with light, to change color or display a new image on a screen.
García-Garibay and colleagues planned to design a crystalline solid with faster-moving components. As a starting point, they considered larger, daily items that they might be able to reproduce at a microscopic scale.
“Two objects we found to be very interesting were compasses and gyroscopes,” said García-Garibay, who also is dean of physical sciences in the UCLA College. “We began to create large-scale models; I literally ordered a few hundred toy compasses and started building structures out of them.”
The researchers found that there were two key factors to imitating a gyroscope or compass at a smaller scale. First, the structure’s exterior case had to be sturdy enough to maintain its shape around typically empty space. Second, the interior rotating component had to be as nearly spherical as possible.
After some trial and error, the team engineered a structure that functioned: a metallo-organic case containing both metal ions and a carbon backbone surrounding a spherical molecule referred to as bicyclooctane. In experiments, the resulting compound — 1.4-bicyclo[2.2.2]octane dicarboxylic acid, a metal-organic framework that the researchers called BODCA-MOF — acted as an amphidynamic material.
Not only that, but computer simulations of the crystal established what the experiments were exhibiting: the constantly-spinning BODCA spheres were each rotating at up to 50 billion rotations per second, as rapidly as they would have in empty space, whether they were rotating counterclockwise or clockwise.
“We were able to use the equations of physics to validate the motions that were occurring in this structure,” said Kendall Houk, UCLA’s Saul Winstein Professor of Organic Chemistry and one of the paper’s authors. “It’s an amazing discovery that you can have extremely rapid motions inside this thing that externally is like a rock.”
Having demonstrated that such a compound can occur, the researchers presently plan to try adding new properties into BODCA-MOF that would allow a magnetic, electric, or chemical stimulus to modify the molecules’ motion.
“The ultimate goal is to be able to control motion in these molecular machines so that we can create materials that respond to external stimuli,” García-Garibay said. That could result in faster computer and electronic displays, he added, or technologies that interact with sonar, radar, or chemicals.
“With such low barriers for rotation, the results mark substantial progress toward freely rotating molecular components embedded in a crystalline matrix, and toward potential functionality,” said Stuart Brown, a UCLA professor of physics and astronomy, and another author of the paper.
The paper’s other authors are Cortnie Vogelsberg, a former graduate student, and Song Yang, a current graduate student, both in UCLA’s chemistry and biochemistry department; Fernando Uribe-Romo of the University of Central Florida; and Andrew Lipton of Pacific Northwest National Laboratory.
The National Science Foundation funded the study.