Jun 1 2018
Russian scientists from the Moscow Institute of Physics and Technology (MIPT), the Technological Institute for Superhard and Novel Carbon Materials (TISNCM), and the National University of Science and Technology MISIS have upgraded the design of a nuclear battery that produces power from the beta decay of nickel-63, a radioactive isotope.
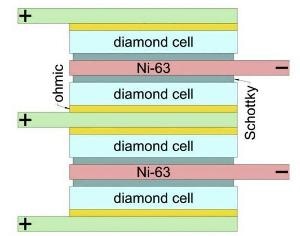 Nuclear battery design. (Image credit: V. Bormashov et al./Diamond and Related Materials)
Nuclear battery design. (Image credit: V. Bormashov et al./Diamond and Related Materials)
The innovative battery prototype developed by the researchers has the ability to pack nearly 3300 mW-hours of energy per gram, which is greater when compared to any other nuclear battery based on nickel-63, and 10 times higher when compared to the specific energy of commercial chemical cells. The study has been reported in the Diamond and Related Materials journal.
Conventional Batteries
In normal batteries that power toys, flashlights, clocks, and other compact autonomous electrical devices, the energy of the well-known redox chemical reactions is used. Here, transfer of electrons from one electrode to the other occurs through an electrolyte. This results in a potential difference between both the electrodes.
Upon connecting the two battery terminals by a conductor, the potential difference is eliminated when the flow of electrons starts, thus producing an electric current. Chemical batteries, also called galvanic cells, possess high power density, or the ratio of the volume of the battery to the power of the produced current.
Yet, chemical cells tend to discharge within a comparatively short period of time, restricting their usage in autonomous devices. Although some of these batteries, known as accumulators, are rechargeable, they have to be replaced for charging. This could be risky, such as in a cardiac pacemaker, or even impossible, if the battery is used for powering a spacecraft.
Nuclear Batteries: History
Luckily, chemical reactions are just one among many probable sources of electric current. In 1913, Henry Moseley was the first to invent a power generator based on radioactive decay. In his nuclear battery, a glass sphere silvered on the inside was equipped with a radium emitter positioned at the center on an isolated electrode.
Electrons emitted as a result of the beta decay of radium led to a potential difference between the central electrode and the silver film. Yet, the device’s idle voltage was very high, of the order of tens of kilovolts, and the current was very low for practical applications.
In 1953, Paul Rappaport hypothesized the application of semiconducting materials for transforming the energy of beta decay into electric power. Beta particles, positrons and electrons, emitted from a radioactive source have the ability to ionize atoms of a semiconductor, producing uncompensated charge carriers.
When a static field exists in a p-n structure, the charges flow in a single direction, leading to electric current generation. Batteries powered by beta decay were termed betavoltaics. The main benefit of betavoltaic cells, when compared to galvanic cells, is their longer life: Since the half-lives of the radioactive isotopes used in nuclear batteries range from tens to hundreds of years, their power output stays almost constant for a very long time.
Sadly, betavoltaic cells have a considerably lower power density when compared to galvanic cells. Without regard to this, betavoltaics were indeed used in the 1970s to power cardiac pacemakers, before being withdrawn to make way for the low-cost lithium-ion batteries, although the lithium-ion batteries have shorter lifetimes.
Betavoltaic power sources are not the same as radioisotope thermoelectric generators (RTGs), which are also known as nuclear batteries yet operate on a distinctive principle. In thermoelectric cells, thermocouples are used for converting the heat released by radioactive decay into electric power. RTGs have limited efficiency, which is dependent on temperature.
However, due to their longer lifetimes and comparatively simple design, thermoelectric power sources are largely used for powering spacecraft such as the New Horizons probe and Mars rover Curiosity. Earlier, RTGs were used on unmanned remote facilities such as automatic weather stations and lighthouses. Over time, this practice was given up since it was hard to recycle used radioactive fuel, which eventually leaked into the environment.
Ten Times More Power
A team of researchers headed by Vladimir Blank, the director of TISNCM and chair of nanostructure physics and chemistry at MIPT, proposed a method for increasing the power density of a nuclear battery by nearly 10 times.
The physicists designed and constructed a betavoltaic battery with nickel-63 as the radiation source and Schottky barrier-based diamond diodes for energy conversion. With the prototype battery, they were able to realize an output power of nearly 1 μW, where the power density per cubic centimeter was 10 1 μW, which is adequate for a modern artificial pacemaker. Since the half-life of Nickel-63 is 100 years, the battery packs nearly 3300 mW-hours of power per gram, which is 10 times more when compared to electrochemical cells.
In the nuclear battery prototype, 200 diamond converters were interlaid with nickel-63 and stable nickel foil layers. The amount of power produced by the converter is based on the thickness of the nickel foil and the converter itself.
This is because both have an impact on the number of beta particles absorbed. At present, the available prototypes of nuclear batteries are poorly upgraded due to their excessive volumes. In case the thickness of the beta radiation source is very high, the electrons emitted by it cannot escape from it. This effect is termed self-absorption.
Yet, when the thickness of the source is reduced, the number of atoms that undergo beta decay in a given unit of time is proportionally minimized. Similar reasoning is applicable to the thickness of the converter.
Calculations First
The aim of the team was to increase the power density of their nickel-63 battery. To achieve this, the passage of electrons through the beta source and the converters was numerically simulated. It was observed that the nickel-63 source is highly effective when its thickness is 2 μm, and the optimal thickness of the converter depending on Schottky barrier diamond diodes is about 10 μm.
Manufacturing Technology
The major technological problem was the fabrication of more number of diamond conversion cells that have a complex internal structure. The thickness of each converter was of the order of only tens of micrometers, such as a plastic bag in a supermarket.
Traditional mechanical and ionic methods of diamond thinning were not appropriate for this task. The scientists from TISNCM and MIPT devised a distinctive technology for fabricating thin diamond plates on a diamond substrate and splitting them off to enable mass-production of ultrathin converters.
The researchers used 20 thick boron-doped diamond crystal plates as the substrate. These plates were grown with the help of the temperature gradient method under high pressure. Ion implantation was employed to produce a 100-nm-thick defective, “damaged” layer in the substrate at the depth of around 700 nm.
A 15-μm-thick boron-doped diamond film was formed on top of this layer with the help of chemical vapor deposition. Then, the substrate was subjected to high-temperature annealing to initiate graphitization of the buried defective layer and recover the top diamond layer.
The damaged layer was removed through electrochemical etching. Once the defective layer was separated through etching, ohmic and Schottky contacts were fitted on the semi-finished converter.
Upon repeating the aforementioned operations, the loss of substrate thickness aggregated to not more than 1 μm per cycle. In total, 200 converters were grown on 20 substrates. This innovative technology is significant from an economic point of view because the cost of high-quality diamond substrates is very high, and hence, it would be impossible to mass-produce converters by substrate thinning.
All converters were connected in parallel in a stack. The technology for rolling 2-μm-thick nickel foil was devised at the Research Institute and Scientific Industrial Association LUCH. Epoxy was used to seal the battery.
The short-circuit current and the open-circuit voltage of the prototype battery are 1.27 μA and 1.02 V, respectively. At 0.92 volts, the maximum output power of 0.93 microwatts is realized.
This power output is in accordance with a specific power of around 3300 mW-hours per gram, which is 10 times more than that observed in commercial chemical cells or the earlier nickel-63 nuclear battery designed at TISNCM.
In 2016, Russian scientists from MISIS had already introduced a prototype betavoltaic battery based on nickel-63. One more working prototype, which was developed at TISNCM and LUCH and demonstrated at the Atomexpo 2017, had a useful volume of 1.5 cm3.
The major drawback in commercializing nuclear batteries in Russia is the lack of nickel-63 production and enrichment facilities. Yet, plans have been proposed to launch the production of nickel-63 on an industrial scale by mid-2020s.
An alternative radioisotope can also be used in nuclear batteries: Radioactive carbon-14 can be used to make diamond converters since it has an exceptionally long half-life of 5700 years. Physicists from the University of Bristol previously reported a study on such generators.
Nuclear Batteries: Prospects
The study reported here could find prospective applications in medical applications. The size of a majority of the sophisticated cardiac pacemakers is more than 10 cm3 and they need around 10 μW of power.
This indicates that it is possible to use the innovative nuclear battery to power up these devices without any major changes to their size and design. “Perpetual pacemakers,” with batteries that need not be serviced or replaced, would enhance patients’ quality of life.
Compact nuclear batteries could also prove highly beneficial, in general, for the space industry. Specifically, there is a demand for autonomous wireless external sensors and memory chips including integrated power supply systems for spacecraft.
Diamond is one of the most radiation-proof semiconductors. Due to its large bandgap, it has the ability to work in a broad range of temperatures, rendering it the perfect material for nuclear batteries that power spacecraft.
The team has proposed to continue its research on nuclear batteries. The researchers have recognized various lines of inquiry that should be sought.
First, battery power can be proportionally increased by enriching nickel-63 in the radiation source.
Second, voltage can be boosted and hence the battery’s power output can be increased at least by a factor of three by developing a diamond p-i-n structure with a controlled doping profile.
Third, the number of nickel-63 atoms in each converter can be increased by increasing the surface area of the converter.
According to Vladimir Blank, TISNCM Director, who is also chair of nanostructure physics and chemistry at MIPT,
The results so far are already quite remarkable and can be applied in medicine and space technology, but we are planning to do more. In the recent years, our institute has been rather successful in the synthesis of high-quality doped diamonds, particularly those with n-type conductivity.
We have decent capabilities for high-quality diamond synthesis, so we are planning to utilize the unique properties of this material for creating new radiation-proof electronic components and designing novel electronic and optical devices.”
Vladimir Blank, TISNCM Director