Dec 20 2018
A newfound protein could be useful in detecting, targeting, and collecting the rare-earth metals used in smartphones from the environment. A detailed description of the protein has been offered by two innovative studies by scientists from Penn State.
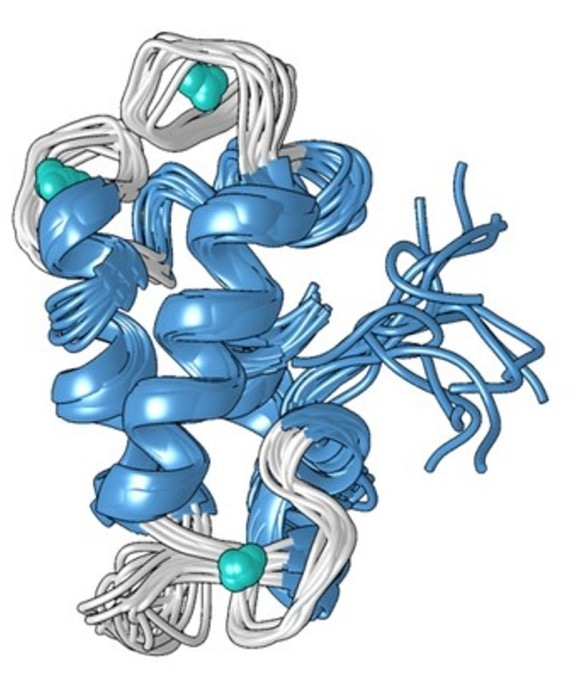 A structural model of the compact metal-bound form of the lanmodulin protein, which is 100 million times better at binding to lanthanides—the rare-earth metals used in smartphones and other technologies—than to other metals like calcium. (Image credit: Penn State)
A structural model of the compact metal-bound form of the lanmodulin protein, which is 100 million times better at binding to lanthanides—the rare-earth metals used in smartphones and other technologies—than to other metals like calcium. (Image credit: Penn State)
Compared to other metals such as calcium, the new protein is 100 million times more efficient in binding to lanthanides—the rare-earth metals used in smartphones and other technologies.
The protein has been described by the first study, published in the Journal of the American Chemical Society, and its distinctive structure, which possibly has a role in its excellent selectivity for lanthanides, has been described in the second paper, which appears online in the Biochemistry journal.
“Recently, there has been a lot of interest in increasing accessibility of rare-earth elements like lanthanides, which are used in the screens and electronics of smartphones, batteries of hybrid cars, lasers, and other technologies,” stated Joseph Cotruvo Jr., assistant professor and Louis Martarano Career Development Professor of Chemistry at Penn State and an author of both studies. “Because the physical properties of rare-earth elements are so similar, it can be difficult to target and collect one in particular. Understanding how this protein binds lanthanides with such incredibly high selectivity could reveal ways to detect and target these important metals.”
The protein, named lanmodulin, was discovered by the researchers within the bacterium Methylobacterium extorquens, which grows in soil and on plant leaves and has a vital role to play in the movement of carbon through the environment. Lanthanides are essential to the bacteria for proper functioning of some of their enzymes, including the one that aids the bacteria in processing carbon, necessary for its growth.
These bacteria need lanthanides and other metals like calcium to grow. They need a way to obtain each metal from the environment and make sure that each goes to the right place within the cell. It appears that these bacteria have developed a unique way to target lanthanides in the environment, where they are much less abundant than other metals like calcium.
Joseph Cotruvo Jr., Assistant Professor and Louis Martarano Career Development Professor of Chemistry, Penn State.
The unique structure of the protein, which was determined by Cotruvo in association with the lab of Scott Showalter, associate professor of chemistry at Penn State, could offer an interpretation for why it is 100 million times more efficient at binding lanthanides over calcium. Cotruvo explained that when the metal is absence, the protein remains mostly unstructured; however, in the presence of the metal, it changes conformation into a compact, well-defined structure.
There are four structures called “EF hands” in the new compact form. In human cells, there are many proteins that contain EF hands, where the hands use calcium for functions such as muscles contracting and neurons firing. Despite the fact that lanthanides are not physiologically relevant in humans and the proteins are just 10 or 100 times more likely to bind lanthanides than they are to bind calcium, these proteins also bind lanthanides. An amino acid known as proline is included in the compact structure of the lanmodulin protein in a unique position in each of the EF hands, thereby likely contributing to the lanthanide selectivity of the protein.
The mechanism of lanmodulin’s selectivity for lanthanides is not yet clear, but we think it comes down to the structural change that occurs in the presence of metals. This structural change is important for the protein’s function; for example, some protein-protein interactions might happen only when the protein is in its compact form. A very small amount of lanthanides is required to induce the conformational change, but it would take much more calcium, more than the bacteria may have, to actually induce the change. This would help to ensure selectivity for lanthanides inside cells.
Joseph Cotruvo Jr., Assistant Professor and Louis Martarano Career Development Professor of Chemistry, Penn State.
Gaining insights into how the protein is selective to such an extent could enable understanding the collection of lanthanides ideal for industrial purposes, including extraction from mining waste streams.
Processing these streams to separate out the rare-earth elements from other metals in an economical way is challenging, both because of the low abundance of the rare earths and their insolubility, requiring large amounts of acid to keep them in solution. Lowering the pH typically lowers affinity toward metals, but we think this protein starts off with such high affinity that it might help overcome these challenges. We are currently investigating this possibility.
Joseph Cotruvo Jr., Assistant Professor and Louis Martarano Career Development Professor of Chemistry, Penn State.
Besides investigating the mechanism of selectivity of the protein and the function of the uniquely located amino acids, the researchers are also studying the affinity of the protein for other metals since lanthanides are usually found along with other metals, such as manganese, iron, and aluminum, which are found in abundance.
We are also very interested in the biological function of the protein. But ultimately we hope that understanding the protein will lead to more environmentally friendly ways to acquire rare-earth metals from the environment.
Joseph Cotruvo Jr., Assistant Professor and Louis Martarano Career Development Professor of Chemistry, Penn State.
Apart from Cotruvo, the research team that initially described the protein includes Emily Featherston, Joseph Mattocks, Jackson Ho, and Tatiana Laremore from Penn State. Apart from Cotruvo and Showalter, the research team that described the structure of the protein includes Erik Cook and Emily Featherston. This study was funded by the Penn State Eberly College of Science Department of Chemistry and the Penn State Huck Institutes of the Life Sciences. The National Science Foundation offered additional support.