By Matt Pullen, B.Sc.Mar 5 2019
An innovative method—discovered by researchers at the Department of Energy’s Oak Ridge National Laboratory, the Drexel University and their associates—could enhance the energy density of conductive two-dimensional (2D) ceramics known as MXenes, which are potential energy-storage materials. The results of the study have been reported in Nature Energy.
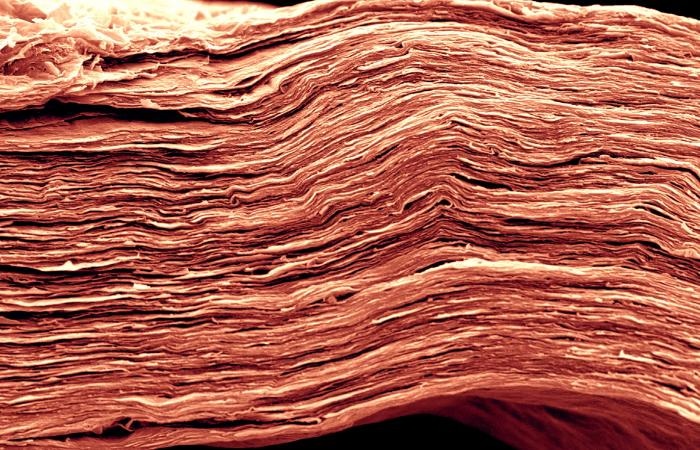 In this MXene electrode, choosing the appropriate solvent for the electrolyte can increase energy density significantly. This scanning electron microscopy image shows fine features of a film only 5 microns thick—approximately 10 times narrower than a human hair. (Credit: Drexel University; image by Tyler Mathis)
In this MXene electrode, choosing the appropriate solvent for the electrolyte can increase energy density significantly. This scanning electron microscopy image shows fine features of a film only 5 microns thick—approximately 10 times narrower than a human hair. (Credit: Drexel University; image by Tyler Mathis)
Present-day batteries, which depend on the charge stored in most of their electrodes, provide high energy-storage capacity; however, slow charging speeds tend to restrict their application in electric vehicles and consumer electronics. Electrochemical capacitors, called supercapacitors, could be the future energy-storage mainstays. These supercapacitors are capable of storing charge at their electrode material’s surface for rapid charging and discharging. Yet, currently, supercapacitors do not have the batteries’ charge-storage capacity, or energy density.
The energy storage community is conservative, using the same few electrolyte solvents for all supercapacitors. New electrode materials like MXenes require electrolyte solvents that match their chemistry and properties.
Yury Gogotsi, Principal Investigator and Professor, Drexel University
Gogotsi planned the research with his postdoctoral researcher, Xuehang Wang.
Different terminal groups, such as fluorine, oxygen, or hydroxyl species, can cover the surfaces of various MXenes. These groups interact powerfully and particularly with diverse solvents as well as dissolved salts present in the electrolyte. A good match between an electrode and electrolyte solvent may subsequently boost the storage capacity or increase the charging speed.
Our study showed that the energy density of supercapacitors based on two-dimensional MXene materials can be significantly increased by choosing the appropriate solvent for the electrolyte. By simply changing the solvent, we can double the charge storage.
Lukas Vlcek, Study Co-Author, University of Tennessee
Vlcek performs research in UT and ORNL’s Joint Institute for Computational Sciences
The study was part of the FIRST (Fluid Interface Reactions, Structures and Transport) Center—an Energy Frontier Research Center headed by ORNL and endorsed by the DOE Office of Science. FIRST research studies the reactions between the fluid and solid interface with consequences for transporting energy in day-to-day applications.
Ke Li from Drexel University developed the titanium carbide MXene from a parent “MAX” ceramic—comprising of titanium (specified by “M”), aluminum (“A”), and carbon (“X”)—and the researcher did this by etching out the aluminum layers to create five-ply MXene monolayers of titanium carbide.
The investigators subsequently soaked the MXenes in lithium-based electrolytes in different solvents that have considerably different molecular properties and structures. Lithium ions carrying the electrical charge easily integrate themselves between the MXene layers.
The materials’ structural integrity before and after electrochemical experiments was revealed by transmission electron microscopy, while the composition and the chemical interactions of MXene between the electrolyte solvent and the MXene surface were characterized by Raman spectroscopy and X-ray photoelectron spectroscopy.
Electrochemical measurements demonstrated that the highest capacitance (the amount of energy stored) was obtained with the help of a less conductive electrolyte.
This is a strange and counterintuitive observation because one would anticipate an oft-used acetonitrile solvent-based electrolyte, possessing the maximum conductivity of all tested electrolytes, to provide the most optimized performance.
While in situ X-ray diffraction revealed the contraction and expansion of the MXene interlayer spacing at the time of charging and discharging upon the use of acetonitrile, no changes were observed in the interlayer spacing upon the use of propylene carbonate solvent. The latter solvent led to relatively higher capacitance. Moreover, electrodes that do not expand upon the entry and exit of ions are also anticipated to endure a greater number of charge-discharge cycles.
In order to study the dynamics of electrolyte solvent media restricted in the MXene layers, the team turned to neutron scattering, which is susceptible to hydrogen atoms present in the solvent molecules.
Lastly, Vlcek performed molecular dynamics simulations, which showed that interactions among the MXene surfaces, electrolyte solvents, and lithium ions strongly rely on the polarity, molecular shape, and size of the solvent molecules. With regards to a propylene carbonate–based electrolyte, a solvent does not surround the lithium ions and thus pack closely between MXene sheets. Yet, in other types of electrolytes, solvent molecules are carried by lithium ions as these ions travel into the electrode, resulting in its expansion upon charging. It is believed that modeling can possibly guide the choice of imminent electrode–electrolyte solvent couples.
Different solvents created different confined environments that they had profound influence on charge transport and interactions of ions with the MXene electrodes. This variety of structures and behaviors was made possible by the layered structure of MXene electrodes, which can respond to charging by easily expanding and contracting the interlayer space to accommodate a much wider range of solvents than electrodes with more rigid frameworks.
Lukas Vlcek, Study Co-Author, University of Tennessee
The paper is titled, “Influences from solvents on charge storage in titanium carbide MXenes.”