Apr 9 2019
Amorphous solids possess an internal structure composed of interconnected structural blocks. These blocks can be quite like the standard structural units found in the equivalent crystalline phase of the same compound. Nearly all identified systems, including metallic alloys or water, can become amorphous when exposed to specific conditions. Specifically, such alloys can exhibit excellent mechanical and physical properties, such as electric conductivity, strength, and corrosion resistance.
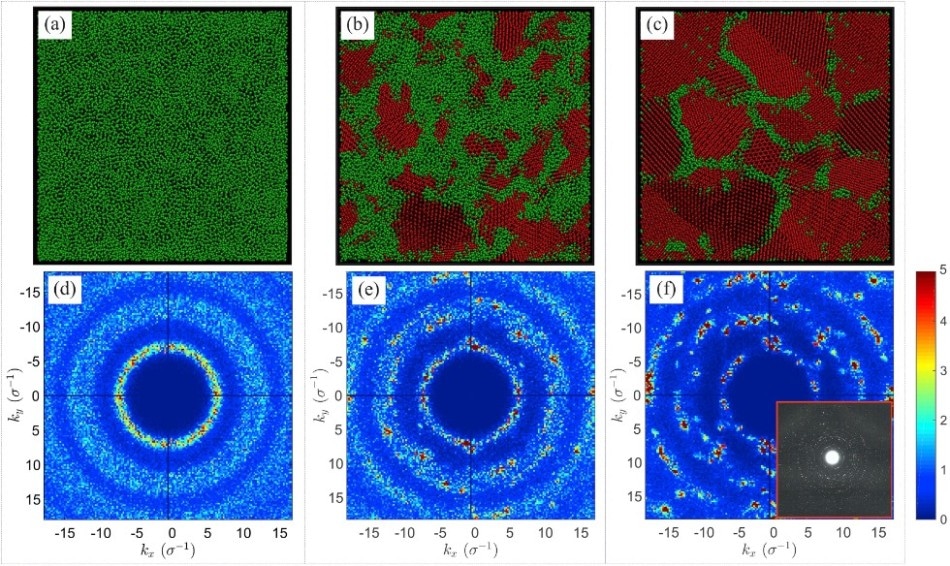 Top panels: snapshots of the system at the temperature and at different times from start of crystallization: (a) glassy system without the crystallization centers; (b) two-phase state of the system containing crystalline nuclei; (c) polycrystalline system consisting of separate crystalline domains. Bottom panels: -projections of the glassy system (d), the two-phase system (e) and the polycrystalline system (f). Inset to panel (f) shows the diffraction pattern obtained by transmission electron microscopy for polycrystalline foil. (Image credit: Kazan University)
Top panels: snapshots of the system at the temperature and at different times from start of crystallization: (a) glassy system without the crystallization centers; (b) two-phase state of the system containing crystalline nuclei; (c) polycrystalline system consisting of separate crystalline domains. Bottom panels: -projections of the glassy system (d), the two-phase system (e) and the polycrystalline system (f). Inset to panel (f) shows the diffraction pattern obtained by transmission electron microscopy for polycrystalline foil. (Image credit: Kazan University)
Combining quantum mechanics and statistical physics with machine learning and Big Data can help find new solutions in physics and materials science. We can now find out many physical properties of a compound just by knowing its chemical composition. We can calculate properties under extremely high temperature or pressure not yet obtainable through actual experiment. This is a part of our approach in this project.
Anatolii Mokshin, Project Head, Kazan University.
In this specific paper, Dr. Mokshin’s group explored the impact of supercooling on the structure and morphology of the crystalline nuclei arising and forming within a liquid metallic film. It was learned that the liquid metallic film at temperatures matching low supercooling levels crystallizes into a monocrystal, while a polycrystalline structure develops at deep supercooling levels. The temperature dependence of critical size of the crystalline nuclei has two distinct regimes with the crossover temperature, which appears because of the particular geometry of the system.