Apr 17 2019
Found in all living organisms, oxidants are byproducts of metabolism and are important for immunity and for healing wounds. However, higher concentrations of oxidants can lead to tissue damage and inflammation.
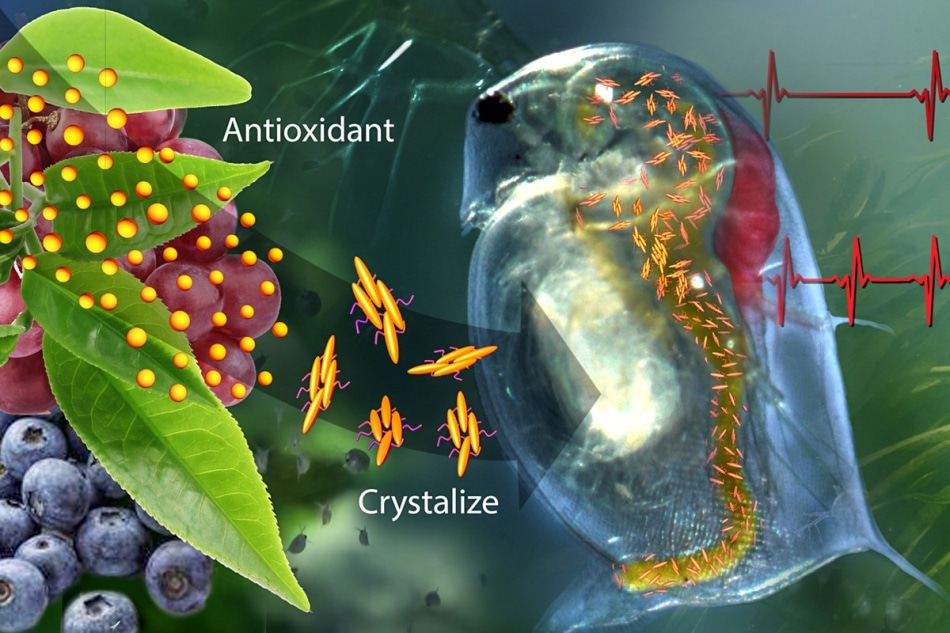 A new drug-delivery system that contains crystalized catechin—an antioxidant found in green tea and fruit—can sense trouble and respond by releasing antioxidant to restore a normal heart rate to water fleas undergoing cardiac stress brought on by high oxidant levels. (Image credit: Janet Sinn-Hanlon, DesignGroup@VetMed, University of Illinois at Urbana-Champaign)
A new drug-delivery system that contains crystalized catechin—an antioxidant found in green tea and fruit—can sense trouble and respond by releasing antioxidant to restore a normal heart rate to water fleas undergoing cardiac stress brought on by high oxidant levels. (Image credit: Janet Sinn-Hanlon, DesignGroup@VetMed, University of Illinois at Urbana-Champaign)
Now, engineers at the University of Illinois have created and tested a unique kind of drug-delivery system that has the ability to sense high concentrations of oxidants and can restore this mild balance by administering the exact amount of antioxidant. The results of the study have been reported in the journal, Small.
Specialized particles and polymers are present in a majority of pharmaceuticals. These components control the concentration or timing of the drug released as soon as it is administered, informed the researchers. Yet, such kinds of additives can obstruct crystallization at the time of the manufacturing phase of certain drugs, such as antioxidants, and cause them to dissolve in the body in an unrestrained way.
We saw an opportunity here to develop a different kind of drug-delivery system that could sense the level of oxidant in a system and respond by administering antioxidant as needed.
Hyunjoon Kong, Study Co-Author and Professor, Department of Chemical and Biomolecular Engineering, University of Illinois.
Together with his team, Kong identified a method to organize crystals of catechin, which are bright green antioxidant present in green tea. He did this with the help of a polymer that is capable of sensing very high concentrations of oxidant.
Next, the scientists tested the responsiveness of the ensuing catechin crystal-containing polymer in Daphnia magna—a common freshwater planktonic crustacean, often known as the water flea.
Heart rate is an indication of the extent to which potentially toxic chemicals influence physiology in water fleas. Daphnids are often used to monitor environmental impacts on ecological systems, and because their hearts are similar to those of vertebrates, they are also used to evaluate the efficacy of cardioprotective drugs.
Hyunjoon Kong, Study Co-Author and Professor, Department of Chemical and Biomolecular Engineering, University of Illinois.
The daphnids were then exposed to water that was contaminated with sublethal concentrations of hydrogen peroxide—a natural oxidant—and, at the same time, their heart rate was also monitored. The researchers observed that based on the concentration of hydrogen peroxide utilized, the mean heart rates of the daphnids dropped from 348 to 290 and 277 beats every minute. When the new crystals of catechin arranged with polymer were added to the experiment, the water fleas were able to recover a heart rate that was close to normal.
While the new polymer shows excellent potential for use in pharmaceuticals, Kong’s team is also exploring its application for reducing the effect of highly oxidizing chemicals in natural waterways.
“Hydrogen peroxide is often used to clean water fouled by excessive algae, and this raises concern about how the oxidant may be affecting living organisms in water,” Kong stated. “We think this new antioxidant-delivery system could be used to address the problem of over-oxidized natural waters.”
The team is planning to go ahead with the development of the polymer for environmental and pharmaceutical applications.
This study proved a concept, but we have more work to do. There is concern over the safety of the specific polymer we used— polyethyleneimine diselenide—but we are getting close to finding a viable replacement.
Hyunjoon Kong, Study Co-Author and Professor, Department of Chemical and Biomolecular Engineering, University of Illinois.
The study was supported by the Korea Institute of Science and Technology-Europe, Department of Defense, the National Institutes of Health, and National Science Foundation.
Stressed cardiac tissue of Daphnia after exposure to hydrogen peroxide
Cardioprotective effects of a catechin-containing drug on Daphnia magna
(Video credit: University of Illinois)