Apr 25 2019
The efficiency of present record holding thermoelectric materials composed of bismuth telluride (Bi2Te3) could be significantly exceeded by tin selenide. Yet, it was believed that the efficiency of tin selenide became huge only at temperatures over 500 °C.
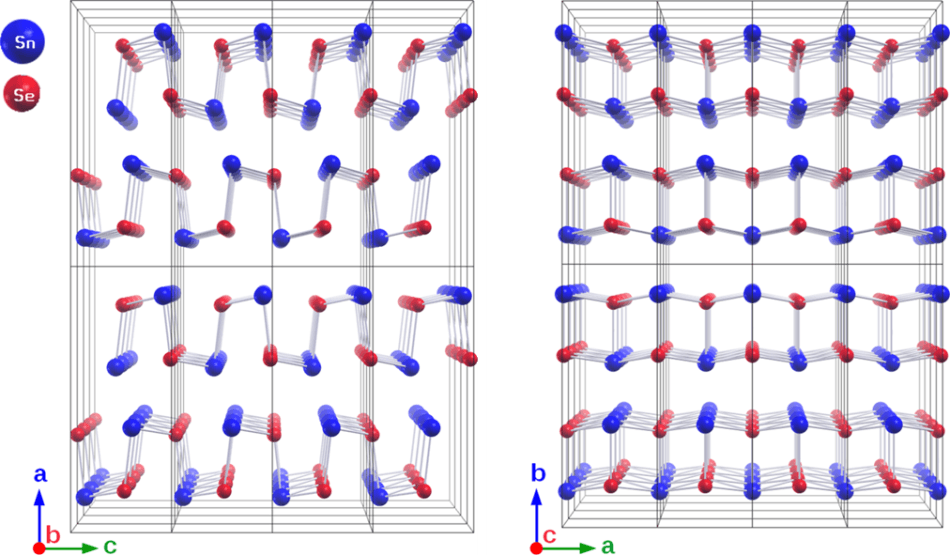 SnSe is a highly layered orthorhombic structure. SnSe undergoes a phase transition of second order at 500 °C with an increase of the crystal symmetry from space group Pnma (left) to Cmcm (right). (Image credit: HZB)
SnSe is a highly layered orthorhombic structure. SnSe undergoes a phase transition of second order at 500 °C with an increase of the crystal symmetry from space group Pnma (left) to Cmcm (right). (Image credit: HZB)
Now measurements made at the PETRA IV and BESSY II synchrotron sources have demonstrated that this compound can also be used as a thermoelectric material at room temperature, as long as high pressure is used.
Since 1821, the thermoelectric effect has been known to researchers—with specific material combinations, a temperature variation produces an electric current. For instance, when waste heat from a combustion engine is used to heat one end of the sample, some part of the energy, which is otherwise lost, can be changed into electrical energy. Yet, in a majority of materials, the thermoelectric effect is very small. This is because a large thermoelectric effect can be achieved only when electrical conductivity is high and heat conduction is poor. Conversely, electrical conductivity and heat conduction are almost invariably closely related.
Owing to this reason, the quest for thermoelectric materials mainly focuses on compounds that have unique crystalline structures like bismuth telluride. So far, one of the best thermoelectric materials known is bismuth telluride; however, both tellurium and bismuth are rare elements and, as a result, their large-scale application is rather limited. Therefore, the quest continues for appropriate thermoelectric materials among non-toxic elements that are more abundantly available.
Six years ago, a team of researchers from the USA found that tin selenide above 500 °C is capable of changing roughly 20% of heat into electrical energy. This efficiency is quite massive and significantly surpasses the value for bismuth telluride. Moreover, selenium and tin are abundantly available.
This very large thermoelectric effect is associated with the re-arrangement or phase transition of tin selenide’s crystal structure. Tin selenide’s crystal structure contains several layers, analogous to puff or filo pastry. At temperatures of 500 °C, the layers begin to self-organize and the heat conduction diminishes, while charge carriers continue to be mobile. To date, no other material has exceeded the efficiency of the thermoelectric effect in tin selenide’s crystallographic orientation.
High pressure works
Headed by Dr Ulrich Schade at the Helmholtz-Zentrum-Berlin (HZB), an international team has now thoroughly examined tin selenide samples using hard X-rays at PETRA IV and infrared spectroscopy at BESSY II. The measurements demonstrate that the preferred crystal structure is created by either high pressure (above 10 GPa) at room temperature or high temperature at normal pressure. In addition, within the high-temperature structure, the electronic characteristics transform from semiconducting to semi-metallic properties. This corresponds with the predictions from hypothetical calculations of the model as well as from band-structure computations.
We are able to explain with our data and our calculations why tin selenide is such an outstanding thermoelectric material over a wide temperature and pressure range.
Dr Ulrich Schade, Helmholtz-Zentrum-Berlin
However, more development work will be required to ensure long-term stability, for instance, before tin selenide-based thermoelectrical devices actually emerge into the market. Then, tin selenide can probably become a readily available and economical alternative to bismuth telluride-based thermoelectrical devices.