Aug 2 2019
In a recent study published in Organic Letters, researchers at Kanazawa University developed a method to generate a highly reactive alkyne, an organic molecule having a C≡C triple bond, from a cyclopropenone, an organic molecule having a strained three membered ring, using a visible light responsive photocatalyst.
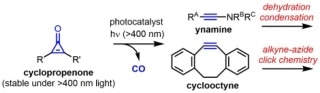 The first photocatalytic active alkyne generation from a cyclopropenone under visible light conditions has been achieved. The generated active alkynes such as ynamine and cyclooctyne can be directly used, without isolation, for the chemical reactions such as a dehydration condensation and alkyne-azide click chemistry.
The first photocatalytic active alkyne generation from a cyclopropenone under visible light conditions has been achieved. The generated active alkynes such as ynamine and cyclooctyne can be directly used, without isolation, for the chemical reactions such as a dehydration condensation and alkyne-azide click chemistry.
Alkynes are a group of organic compounds that are used to manufacture industrial reagents and polymers. Photolysis of a cyclopropenone with UV-light is a useful method to generate a highly reactive alkyne. However, if the reaction mix contains accompanying compounds that are sensitive to UV light, they will degenerate. Therefore, performing this reaction in the presence of visible light instead will keep such compounds intact. A research team at Kanazawa University has identified catalysts that can facilitate the photolysis of visible-light-stable cyclopropenones under visible-light conditions.
The team first screened a panel of six potential catalysts to identify those yielding the highest proportion of aminoalkynes from aminocyclopropenones in the presence of visible light. To assess this, a phototriggered dehydration condensation reaction was set up. This is one of the few reactions wherein the aminoalkyne synthesized from the aminocyclopropenone directly undergoes a series of intermediate steps to yield chemical compounds known as amides.
When this reaction mixture was irradiated with a household fluorescence lamp, two catalysts showed the highest yield of the amide. In the presence of these two catalysts more than 90% of the cyclopropenone was consumed, suggestive of their efficiency under visible light. These observations were not seen under dark conditions, further suggesting the dependence of these two catalysts on light within the visible spectrum. Interestingly, the researchers also noticed that these two catalysts seemed to have the high reduction potential at their excited states and low reduction potential at their ground states. This redox potential possibly causes the excited catalysts to snatch an electron away from cyclopropenone, initiating the reaction chain. The oxidized cyclopropenone would be unstable and decompose to the ring-opened radical cation. The radical cation would receive one-electron from the photocatalyst and then generate carbon monoxide and the active alkyne. The mechanism of visible-light activated catalysts is thus thought to be different from catalysts that function under UV light.
To test the practical application of these catalysts, the reaction was then performed in the presence of tetrazole, a highly UV-sensitive molecule. As expected, under UV light conditions, tetrazole decomposed, yielding only 46% of the amide. On the other hand, under visible light not only was the tetrazole found to be intact, but the yield of the amide was much higher: almost 75%.
This study proposed a novel method of alkyne synthesis without dependence on UV light. “[We] have developed a novel phototriggered active alkyne generation reaction with a visible-light-responsive-photocatalyst. The method would be especially useful when UV light cannot be used for alkyne generation due to the UV light-sensitivity of other coexisting substrates,” conclude the researchers.
Background:
Alkynes and their synthesis: Alkynes are unsaturated organic molecules consisting of at least one carbon-carbon triple bond and carbon chains of varying length. While there are several methods of synthesizing alkynes, photolysis of cyclopropenone produces unstable alkynes which are directly used in other chemical reactions, eliminating the need for their isolation. Due to the presence of triple bonds, many different functional groups can be added to alkynes resulting in halogenated, hydrogenated and even oxidized products. Thus, optimizing alkyne synthesis has great industrial value in producing different chemical reagents.
Reference:
Kenji Mishiro, Takeshi Kimura, Taniyuki Furuyama, Munetaka Kunishima. Phototriggered Active Alkyne Generation from Cyclopropenones with Visible Light-Responsive Photocatalysts. Organic Letters, 2019.