Aug 20 2019
NUS chemists have developed a catalytic method using visible light for the difunctionalisation of ethylene for potential use in the production of fine chemicals.
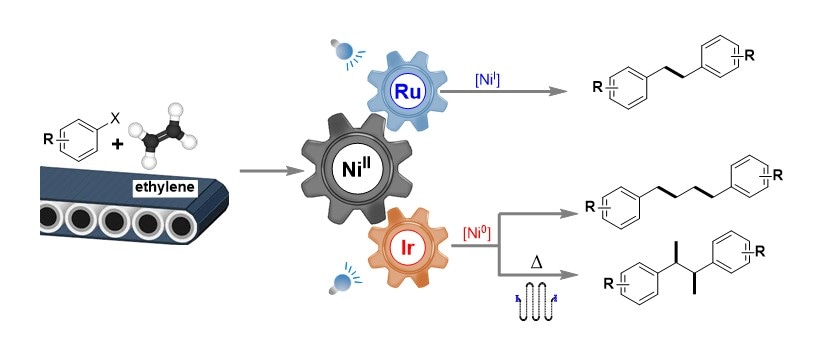 Illustration showing the nickel catalysed reactions between commercially available aryl halide and ethylene. The oxidation state of the nickel catalyst changes to +1 or 0 when it is modulated by ruthenium- or iridium-based photocatalyst to produce the associated chemical products. For the reaction involving the iridium-based photocatalyst, the presence of heat in a “stop flow” reactor results in a different chemical structure.
Illustration showing the nickel catalysed reactions between commercially available aryl halide and ethylene. The oxidation state of the nickel catalyst changes to +1 or 0 when it is modulated by ruthenium- or iridium-based photocatalyst to produce the associated chemical products. For the reaction involving the iridium-based photocatalyst, the presence of heat in a “stop flow” reactor results in a different chemical structure.
Ethylene is a feedstock which is widely used in the chemical industry to produce a wide range of products. It has an estimated annual production of more than 150 million tonnes, far exceeding that of any other organic compound. Despite its availability, there are relatively few established methods of using ethylene to produce high value fine chemicals. The existing methods of converting ethylene into more complex molecules for fine chemicals are limited to monofunctionalisations, which involve the modification of a single functional group. More effective catalytic methods incorporating difunctionalisation of ethylene are highly desirable.
A research team led by Prof WU Jie, from the Department of Chemistry, NUS has developed a process using visible light together with photoredox and nickel catalysts to enable the difunctionalisation of ethylene gas to produce a wide variety of chemical compounds. By using a different set of reaction parameters, they managed to produce 1,2-diarylethanes, 1,4-diarylbutanes and 2,3-diarylbutanes from ethylene gas in a highly selective manner. The selectivity is achieved by the modulation of the nickel catalyst by ruthenium- or iridium-based photocatalysts with different reductive potentials and the presence of heat in a “stop flow” reactor. Although ethylene has a tendency to form long polymer chains, their method is able to avoid this issue through coordination with the metal catalyst. This prevents the formation of free radical species, which leads to long chain polymerisation of ethylene. The synthetic method provides a direct way to transform a widely available chemical feedstock into more complex molecules for use in the chemical industry.
Prof Wu said, “The formation of two or four carbon linkers derived from ethylene cannot be easily obtained directly by other synthetic means. The number of ethylene molecules which can be added to the resulting product is governed by the oxidation state of the nickel catalysts. This is modulated by the photoredox catalyst. Nickel catalysts with an oxidation state of +1 and 0 facilitated the addition of one or two ethylene molecules respectively.”
“This type of photoredox-modulated catalytic reaction for obtaining chemical products is a new development in the field of organic synthesis,” added Prof Wu.
The research team plans to develop more advanced catalytic processes for the development of fine chemicals using ethylene feedstock under visible light irradiation.