Nov 18 2019
A low-temperature catalyst for eliminating NOx gas from industrial exhaust using ammonia has been created by researchers at the Tokyo Metropolitan University.
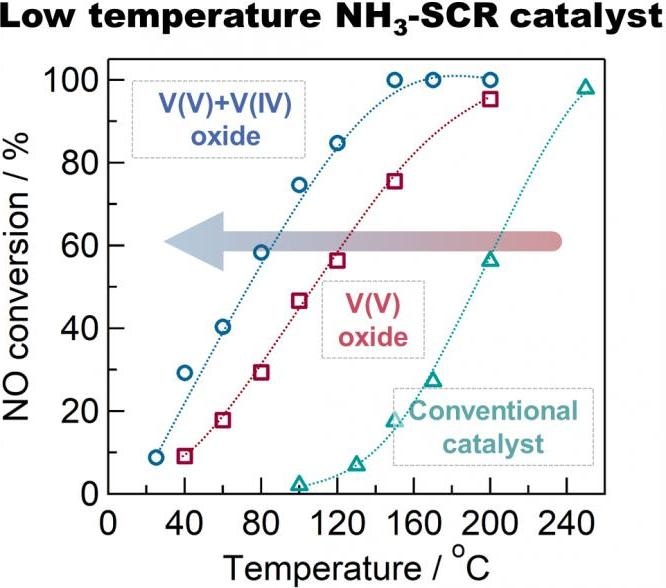 Conversion rate of nitrogen oxides at different temperatures for conventional, V(V) oxide and V(IV) + V(V) oxide “defective” catalysts. The mixture of V(V) and V(IV) oxides showed a 10-fold improvement in the 100–150 °C range. Image Credit: Tokyo Metropolitan University.
Conversion rate of nitrogen oxides at different temperatures for conventional, V(V) oxide and V(IV) + V(V) oxide “defective” catalysts. The mixture of V(V) and V(IV) oxides showed a 10-fold improvement in the 100–150 °C range. Image Credit: Tokyo Metropolitan University.
Made up of bulk “defective” vanadium oxide rather than vanadium oxides supported on titanium oxide, which is the case with commercial catalysts, the catalyst functions at lower temperatures (<150 °C) with much greater efficiency.
The researchers showed an evident enhancement in performance, and detected the reaction mechanisms contributing to the difference.
Some of the common atmospheric pollutants produced by burning coal, fossil fuels, and natural gas are nitrogen monoxide (NO), nitrogen dioxide (NO2), or oxides of nitrogen (NOx). They are a leading cause of photochemical smog and acid rain, which makes their elimination from factory and vehicle emissions very critical.
A core technology for eliminating nitrogen oxides is their reaction with ammonia via selective catalytic reduction (SCR), where NOx is made harmless through reduction to water and nitrogen. Vanadium oxides supported on titania are said to possess superior selectivity for conversion to nitrogen, and have been effectively applied to stationary boilers.
However, a major bottleneck for supported catalysts is the high temperature necessary for catalytic activity, generally 200 °C to 400 °C. This generally results in units being positioned close to, for example, the boiler in power plants, where they can be easily impaired not only physically by ash but by the build-up of ammonium sulfates.
These disabling factors can be avoided if the unit is positioned downstream after an electrostatic precipitator for eliminating dust and a desulfation system to eliminate sulfate deposits.
However, this method necessitates high catalytic activity at lower temperatures, since the temperature of the exhaust gas has usually dropped to about 100 °C by this time. A catalyst that functions at lower temperatures is needed.
A research team headed by Yusuke Inomata and Toru Murayama from Tokyo Metropolitan University has now created a catalyst based on bulk vanadium oxides. Vanadium (V) oxide (V2O5) is a regularly occurring state of vanadium oxide; the team, however, effectively synthesized a blend of vanadium (V) and vanadium (IV) oxides, or “defective” vanadium oxide, by heating a precursor to 270 °C.
The researchers discovered that this “defective” catalyst had superior catalytic activity at temperatures down to 100 °C. At this temperature, the speed at which NOx is changed to harmless nitrogen was 10 times quicker than traditional titania-supported vanadium oxide catalysts, displaying excellent performance where traditional catalysts fall short.
The enhancement was credited to the presence of V(IV) which forms “Lewis acid” (electron-accepting) sites, boosting the reaction of nitrogen oxide with ammonia to turn into nitrogen.
Apart from practical application to industrial catalysis, the team anticipates that the mechanisms they have discovered serve as a model system for additional scientific studies.
This study was a collaborative work with the Chugoku Electric Power Company, Incorporated, and was partly funded by the Cooperative Research Program of the Institute for Catalysis, Hokkaido University (Grant #19B1016).