Jun 1 2020
Although chemists often make use of high temperatures to achieve things, at times, keeping cool can work wonders. KAUST scientists have found that a low-temperature method can be useful to make improved single crystals for solar cells.
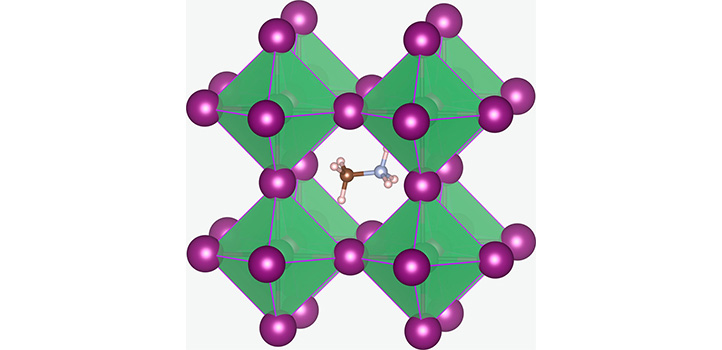 The crystal structure of methylammonium lead halide, the material used to make the solar cell. Image Credit: Reproduced under a creative commons license © Eames, C., Frost, J., Barnes, P. et al. Ionic transport in hybrid lead iodide perovskite solar cells. Nature Communications 6, 7497 (2015).
The crystal structure of methylammonium lead halide, the material used to make the solar cell. Image Credit: Reproduced under a creative commons license © Eames, C., Frost, J., Barnes, P. et al. Ionic transport in hybrid lead iodide perovskite solar cells. Nature Communications 6, 7497 (2015).
At the KAUST Catalysis Center, Osman M. Bakr’s group has been discovering several innovations in perovskites—materials that exhibit high potential for the development of new generations of solar cells.
Novel perovskites have both negative and positive ions in an arrangement similar to that found in the natural perovskite calcium titanate (CaTiO3). Lead halide perovskites that include halide ions and lead ions, like iodine and chlorine in the perovskite mix, have been gaining considerable attention for their optoelectronic applications.
Halide perovskite solar cells are considered the fastest growing photovoltaic technology.
Abdullah Alsalloum, Study Co-First Author and Masters Student, KAUST
The crystals have been produced at high temperatures, but such crystals have created disturbing faults. At present, the KAUST researchers have come up with a solution, in both senses of the word, which enables developing better crystals.
In general, their “solvent engineering” method alters the liquid solvent in which the perovskite components are dissolved at the beginning, and in which they join to form the perovskite crystals.
Researchers are developing a particular perovskite known as methylammonium lead iodide, including large methylammonium ions (CH6N+), combined with iodide (I−) and lead (Pb2+) ions.
Earlier, it was essential to maintain the solutions used to make the crystals at 120 °C, but such a high temperature hindered with the maximum integration of iodide and methylammonium ions into the structure.
We investigated a variety of different solvents to carry the perovskite components and allow them to crystallize. Eventually, we found a solution that could work at less than 90 degrees Celsius and produce single crystals that were of significantly higher quality.
Bekir Turedi, Study Co-First Author, KAUST
The improvement in the formation of crystals corresponded to enhanced performance upon using the crystals as the light-absorbing layers in solar cells.
We obtained markedly higher photovoltages, and power conversion efficiencies approaching 22 percent, among the highest ever reported for these perovskites.
Osman M. Bakr, KAUST
The team confirmed that the new single crystals are, at present, too small to be used for commercial applications—an issue that now impedes several other efforts to improve the complete potential of perovskites in solar cells.
However, this new study at KAUST is an essential first step toward addressing the problem of size as well as further enhancements.
Alsalloum concludes, “The field is still in its infancy and the most exciting parts are yet to come.”
Journal Reference:
Alsalloum, A. Y., et al. (2020) Low-Temperature Crystallization Enables 21.9% Efficient Single-Crystal MAPbI3 Inverted Perovskite Solar Cells. ACS Energy Letters. doi.org/10.1021/acsenergylett.9b02787.