Nov 5 2020
Electric vehicles (EVs) exhibit immense potential for an energy-efficient, sustainable future. However, one of their drawbacks is the lack of a durable, high-energy-density battery that minimizes the need to fuel up on long trips.
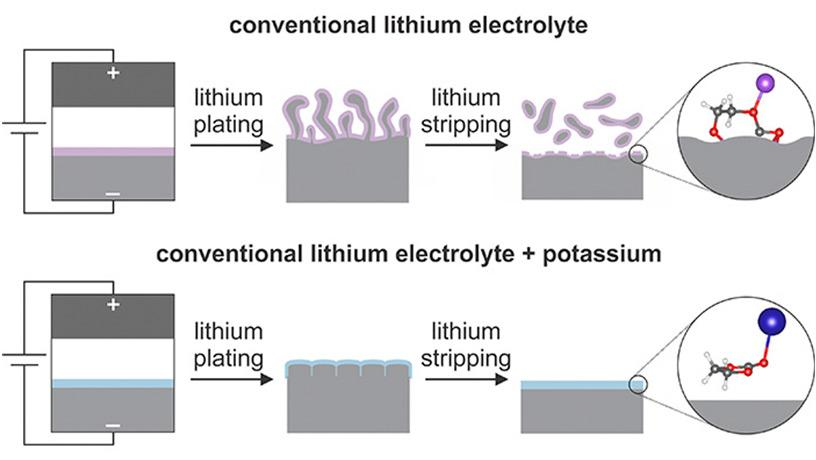 The team used nuclear magnetic resonance imaging and computer simulations to better understand the reactivity and structure of molecules on the surface of lithium metal anodes that could lead to improved performance.
The team used nuclear magnetic resonance imaging and computer simulations to better understand the reactivity and structure of molecules on the surface of lithium metal anodes that could lead to improved performance.
The same holds true for houses at the time of blackouts and power grid failures—small, efficient batteries that can power a home for over one night without electricity have not yet been developed.
Although state-of-the-art lithium batteries that are durable, lightweight, and offer economical energy storage could transform the industry, several difficulties have hampered successful commercialization.
A crucial problem is that while rechargeable lithium metal anodes play a vital role in the optimum performance of this new wave of lithium batteries, during operation, they are highly vulnerable to the accumulation of dendrites and microstructures that can result in fire, hazardous short-circuiting, and even explosion.
At Columbia Engineering, scientists recently reported that they have identified that alkali metal additives, like potassium ions, can avoid the proliferation of lithium microstructures when the battery is in use.
They utilized a combination of microscopy, nuclear magnetic resonance (similar to an MRI), and computational modeling to determine that the addition of tiny amounts of potassium salt to a traditional lithium battery electrolyte leads to special chemistry at the lithium or electrolyte interface. The study was recently published online in the Cell Reports Physical Science journal.
Specifically, we found that potassium ions mitigate the formation of undesirable chemical compounds that deposit on the surface of lithium metal and prevent lithium ion transport during battery charging and discharging, ultimately limiting microstructural growth.
Lauren Marbella, Principal Investigator and Assistant Professor of Chemical Engineering, School of Engineering and Applied Science, Columbia University
Marbella group’s finding that alkali metal additives inhibit the development of non-conductive compounds on the surface of lithium metal varies from conventional electrolyte manipulation methods, which involve depositing conductive polymers on the surface of the metal.
The study is considered as one of the first comprehensive characterizations of the surface chemistry of lithium metal utilizing NMR and illustrates the power of this method to design new electrolytes for lithium metal.
Marbella’s findings were complemented with density functional theory (DFT) calculations carried out by collaborators in the Viswanathan group in mechanical engineering at Carnegie Mellon University.
Commercial electrolytes are a cocktail of carefully selected molecules. Using NMR and computer simulations, we can finally understand how these unique electrolyte formulations improve lithium metal battery performance at the molecular level. This insight ultimately gives researchers the tools they need to optimize electrolyte design and enable stable lithium metal batteries.
Lauren Marbella, Principal Investigator and Assistant Professor of Chemical Engineering, School of Engineering and Applied Science, Columbia University
Currently, the researchers are testing alkali metal additives that prevent the growth of detrimental surface layers in combination with more conventional additives that promote the development of conductive layers on lithium metal. Moreover, they are actively making use of NMR to instantly quantify the rate of lithium transport via this layer.
Journal Reference:
May, R., et al. (2020) Leveraging Cation Identity to Engineer Solid Electrolyte Interphases for Rechargeable Lithium Metal Anodes. Cell Reports Physical Science. doi.org/10.1016/j.xcrp.2020.100239.