Dec 1 2020
Human life has been transformed by plastics, pharmaceuticals, and several other chemical products. To make such products, chemists frequently make use of a catalyst—often based on rare metals—at different points in their syntheses.
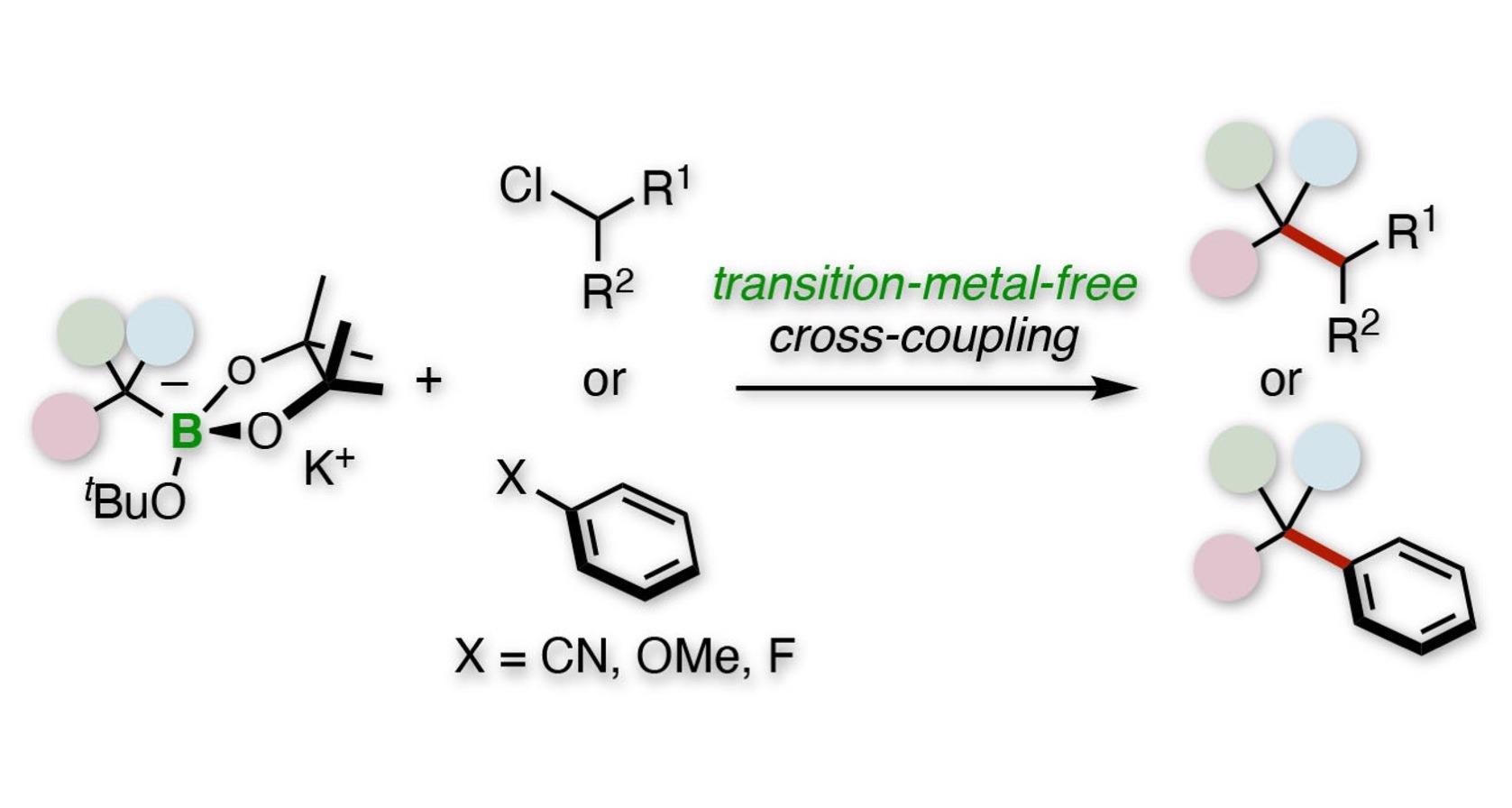 Tertiary alkylative cross-coupling of alkyl or aryl electrophiles. Image Credit: Kanazawa University.
Tertiary alkylative cross-coupling of alkyl or aryl electrophiles. Image Credit: Kanazawa University.
Rare-metal catalysts are extremely beneficial but their restricted supply indicates that their use cannot be sustainable in the long term. Therefore, synthetic chemists have been looking for an alternative.
Such an alternative has been described by scientists from Kanazawa University in a study published recently in the Angewandte Chemie journal. Their study on a wide class of chemical reactions commonly used in pharmaceuticals and other syntheses will lay the basis for a more sustainable chemical sector.
The 2010 Nobel Prize in Chemistry was awarded to scientists who made use of palladium metal-based catalysts to execute a usual chemical reaction called cross-coupling.
These catalysts function very well to synthesize the so-called congested quaternary carbon centers, which are commonly found in molecules utilized in medicine and agriculture. But to ensure long-term sustainability, scientists required a substitute for rare-metal catalysts.
We used benzylic organoborates to perform tertiary alkylative cross-coupling of aryl or alkyl electrophiles. Our procedure does not use rare elements and is a straightforward route to quaternary carbon centers.
Hirohisa Ohmiya, Study Corresponding Author, Kanazawa University
Initial studies by the researchers included a tertiary benzylboronate that is first triggered by a potassium alkoxide base to turn into a benzyl anion. Then, this anion experiences a cross-coupling reaction with a secondary alkyl chloride electrophile.
The reaction has broad scope. For example, replacing the phenyl group of the boronate with various aromatic rings was successful, and the electrophile can be a wide range of rings and linear chains.
Hirohisa Ohmiya, Study Corresponding Author, Kanazawa University
During further studies, the secondary alkyl chloride was replaced with several aryl fluorides, aryl ethers, and aryl nitriles. Most of these reactions were successful, including those with 4-fluorophenylbenzene and 4-cyanopyridine.
A comment in the Nature journal on November 19th, 2020, shows that the COVID-19 pandemic has interrupted the supply chains to several rare metals that are vital to the chemical sector.
Hundreds of factories and mines have been closed, and several national borders are highly restricted than before the pandemic. A long-standing solution to supply chain disruptions is to design synthetic protocols that are not based on rare metals.
This study is an essential part of that measure and will help make chemical syntheses more sustainable for the generations to come.
Journal Reference:
Takeda, M., et al. (2020) Transition-Metal-Free Cross-Coupling by Using Tertiary Benzylic Organoboronates. Angewandte Chemie. doi.org/10.1002/anie.202010251.