Dec 8 2020
A fascinating discovery that dihydrogen phosphate anions—inorganic ions essential for cellular activity—tend to bind with other dihydrogen phosphate anions despite being charged negatively was made by researchers from the University of New South Wales (UNSW) Sydney in collaboration with researchers from Western Sydney University and The Netherlands.
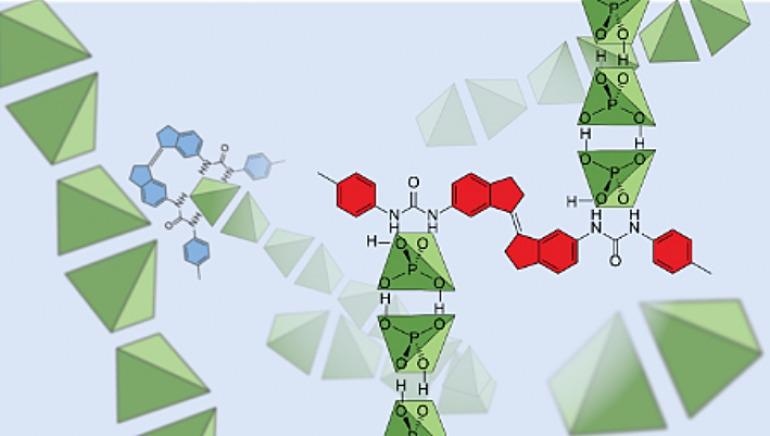 Representation showing dihydrogen phosphates (in green) binding together in solution. Image Credit: University of New South Wales Sydney.
Representation showing dihydrogen phosphates (in green) binding together in solution. Image Credit: University of New South Wales Sydney.
The same researchers created a molecule with the ability to “grab” these dihydrogen phosphate anions and, based on the color of the light shone on them, either increase or hinder their movement in solution.
Published recently in The Journal of the American Chemical Society, the study offers a better understanding of molecular interactions that take place at the time of biochemical processes, while offering new techniques to regulate the transport of molecules in solution.
According to Jon Beves, an associate professor from UNSW’s school of chemistry, chemists have always been familiar that dihydrogen phosphate was “a bit weird” and difficult to study in solution. However, to date, it was not clear what was actually happening.
Our work shows that these negatively charged anions are actually bound together, even in dilute solutions where hydrogen bonds are thought to be extremely weak.
Jon Beves, Associate Professor, School of Chemistry, University of New South Wales
“The hydrogen bonds between dihydrogen phosphate anions seem to be surprisingly strong. They’re strong enough to overcome like-charge repulsion, and strong enough to hold the anion clusters together even when dissolved in hydrogen-bonding solvents that we expected would tear them apart,” added Beves.
Beves states that the new insight could also elucidate somewhat the structure of biological membranes, or how DNA or RNA are attracted to one another in solution, since all such interactions involve phosphate groups.
Moreover, the ability to regulate the movement of such molecules in solution with the help of light gives rise to some interesting concepts on how this could be employed in environmental or biological conditions.
Mixed liquid solutions are made up of lots of molecules all moving and tumbling at random. This makes it really hard to do things like extract valuable or polluting metals from dilute solutions or deliver drug molecules where they need to go in a human body. If we could control the movement of some of those molecules and tell them where to go, it could make those tasks much more achievable.
Jon Beves, Associate Professor, School of Chemistry, University of New South Wales
However, Beves highlights that these applications would be farfetched and necessitate far more research. Currently, Beves is excited about doing essential work in a poorly perceived area of basic chemistry.
According to Beves, the work performed by his team involved the use of an organic solvent known as dimethyl sulfoxide, and he hopes that future studies would analyze whether phosphate behaves similarly in water, where all biological chemistry occurs.
However, as a next step forward, his team endeavors to investigate how molecules can be transported actively in solution.
Our next goals will be to use these types of interactions to actively drive the transport of molecules using light—for example, using a laser pointer to direct molecules to move.
Jon Beves, Associate Professor, School of Chemistry, University of New South Wales
Journal Reference
MacDonald, T. S. C., et al. (2020) Controlled Diffusion of Photoswitchable Receptors by Binding Anti-electrostatic Hydrogen-Bonded Phosphate Oligomers. Journal of the American Chemical Society. doi.org/10.1021/jacs.0c09072.