Feb 9 2021
Lithium metal batteries (LMBs) could double the amount of energy stored in lithium-ion batteries if their anodes are prevented from disintegrating into small pieces during use.
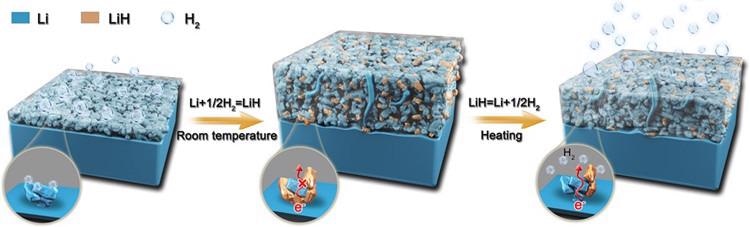 The temperature-sensitive equilibrium governing the formation and decomposition process of LiH at Li anode. Image Credit: Gaojie Xu.
The temperature-sensitive equilibrium governing the formation and decomposition process of LiH at Li anode. Image Credit: Gaojie Xu.
Headed by Prof. Guanglei Cui from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS), a team of scientists has found what makes LMBs to 'self-destruct' and suggested a technique to prevent it. The study results were published in Angewandte Chemie on January 19th, 2021.
The study paves the way for radically improving the energy stored in batteries without increasing their size, while reducing the cost.
Although LMBs were the original idea behind durable batteries, their anodes disintegrate into small pieces—a microstructure termed 'pulverization.' Thus, the LMBs quickly stop functioning when they are cycled through.
In reality, lithium-ion batteries were a compromise—modifying the LMB concept avoided the anode failure by making use of a graphite anode, but provided very low energy storage levels.
One of the challenges in developing LMBs is a lack of insights, and even a debate over, why the anode fails. Traditionally, it is said that small branchlike structures of lithium, known as dendrites, form during battery cycling. The pulverization structure always appears in any failed LMB.
Whether lithium hydride (LiH) occurs in the pulverization structure is a controversial question. LiH exhibits poor electrical conductivity, while being highly brittle, which would account for the pulverization. A group of researchers had previously found LiH as a unique kind of dendrite, but another group discovered no such findings.
But both the groups had employed only simplified versions of an LMB. The QIBEBT researchers operated a practical LMB under standard operating conditions to accurately analyze what happens during battery cycling.
They used a kind of mass spectrometry (an analytical tool for the identification of unknown compounds) to confirm that LiH in fact becomes the dominant compound on the anode during battery usage.
However, most significantly, it was discovered that this chemical reaction is sensitive to temperature: it occurs only at room temperature, and the process is reversible if the temperature increases above this level.
This indicates ways by which LiH production can be avoided, either through heat treatment or a pressure treatment that offers the same effect, or a combination of both. Other options are to inhibit the production of hydrogen ions, or the addition of interface materials with the ability to safeguard the lithium from the hydrogen.
Coming out of this study, the next step is to produce some form of really good lithium protection, which should then deliver on the long-held promise of practical applications of the ‘holy grail’ of lithium metal batteries.
Guanglei Cui, Study Lead Author and Scientist, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Journal Reference:
Xu, G., et al. (2021) The formation/decomposition equilibrium of LiH and its contribution on anode failure in practical lithium metal batteries. Angewandte Chemie. doi.org/10.1002/anie.202013812.