Feb 25 2021
In the modern world, several materials―ranging from the plastics dominating the globe to the electronic chips that power it―are made of polymers.
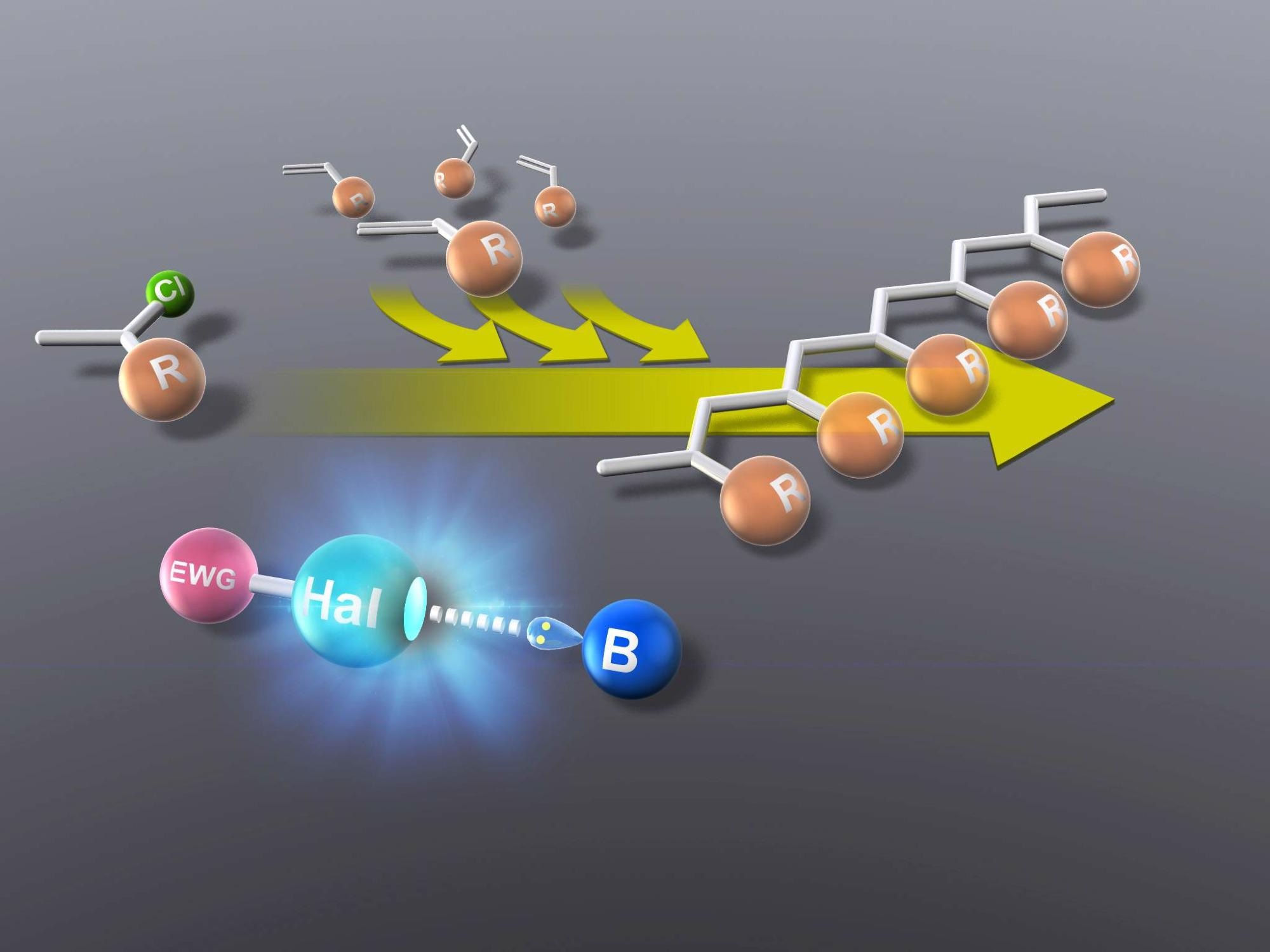 Simpler vinyl polymerization with novel halogen-based catalyst. Image Credit: Nagoya Institute of Technology.
Simpler vinyl polymerization with novel halogen-based catalyst. Image Credit: Nagoya Institute of Technology.
Their ubiquity and the changing demands of the world have made the quest for improved and more efficient methods for manufacturing polymers an ongoing research concern. Existing environmental problems require the application of methods and input materials that are eco-friendly.
A new study by researchers from Nagoya Institute of Technology, Japan, deals with this issue, introducing a new twist to a polymerization method that has existed and has been successful from the 1980s: living cationic polymerization, in which the polymer chain growth lacks the ability to cease until the monomer is used up.
For the first time, the researchers have demonstrated metal-free organocatalysis for this reaction at ambient temperature for styrene and vinyl polymers—two of the most ubiquitous polymers used in plastics. Apart from being more efficient compared to existing metal-based methods, their method is also ecofriendly.
The study results have been reported in the Polymer Chemistry journal from the Royal Society of Chemistry.
As part of the study, the researchers first investigated the usability of non-ionic and multidentate (or various electron-pair accepting) halogen bonding organocatalysts, particularly two iodine-carrying polyfluoro-substituted oligoarenes, for the living cationic polymerization of isobutyl vinyl ether.
Dr. Koji Takagi, lead scientist in the study, explained one of their reasons for selecting this.
The non-ionic characteristic is advantageous because the catalyst is soluble in less polar solvents like toluene which is more suitable for such polymerization of vinyl monomers.
Dr Koji Takagi, Study Lead Scientist, Nagoya Institute of Technology
The researchers observed that in the presence of tridentate variant, the reaction progressed smoothly even at ambient temperature, thus generating good yield―although less than the theoretical limit―in a reasonable time. This was achieved without the decomposition of the catalyst or the catalyst appearing as an impurity in the product.
Dr Takagi explained that this could be a benefit over current metallic catalysts used in the industry.
While metal-based catalysts have significantly contributed to the materials sciences over the past century, the contamination of remaining metallic impurities often brings about a decrease in the produced materials’ lifetime and performance. We believe that the present finding will lead to the production of highly pure and reliable polymeric materials.
Dr Koji Takagi, Study Lead Scientist, Nagoya Institute of Technology
While mentioning this, he is also referring to the other major finding of the study.
In the second part of their study, the researchers assessed the usability of ionic iodoimidazolium catalysts with different counter anions (the negative ions that accompany the positively charged group) for the polymerization of p-methoxystyrene (pMOS) and unsubstituted styrene, where the latter is harder to polymerize than the former.
pMOS was found to polymerize easily at ambient temperature within 2 hours and without any catalyst decomposition of a bidentate 2-iodoimidazolium salt with a triflate counter anion. Unsubstituted styrene offered the highest polymer yield through a reaction at −10°C for 24 hours with a bulky and anion-stabilizing counter ion-containing catalyst.
Dr Takagi reported the details of the yielded products as follows:
Although the obtained polymers are not intended for any specific purpose, our methodology is expected to be applied to the synthesis of conductive polymers and degradable polymers, which should not include metallic impurities if they’re to be constructed for practical use.
Dr Koji Takagi, Study Lead Scientist, Nagoya Institute of Technology
The study results are crucial for progressing with the more efficient synthesis of polymeric materials for a range of applications. But the successful use of organocatalysts at ambient temperature also provides various other benefits.
Firstly, organocatalysts are not sensitive to oxygen and moisture, thereby solving the sometimes serious issue that the comparatively hygroscopic nature of ionic catalysts causes in the case of such controlled polymerization reactions. They are readily available and thus inexpensive.
They are not hazardous to the environment as well. When reactions are performed at ambient temperature, the energy demands are low.
Thus, this study is opening the door for low-cost electronics in the future made of ecofriendly materials in sustainable ways.
Halogen bonding organocatalysts (R-Hal-B) enabled seamless living cationic polymerization of vinyl (R-Cl) monomers at ambient temperature, generating a good quantity of pure yield, paving the way to realizing cost-effective eco-friendly vinyl polymerization reactions for the industry.
This study was financially supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (18K05215).
Journal Reference:
Takagi, K., et al. (2021) Cationic polymerization of vinyl monomers using halogen bonding organocatalysts with varied activity. Polymer Chemistry. doi.org/10.1039/D0PY01207F.