Apr 21 2021
When it comes to fuel cell technology, protons are considered the next big thing. The subatomic exchange generates significant power that challenges modern solid-state fuel cell technology that is currently used to fuel space shuttles.
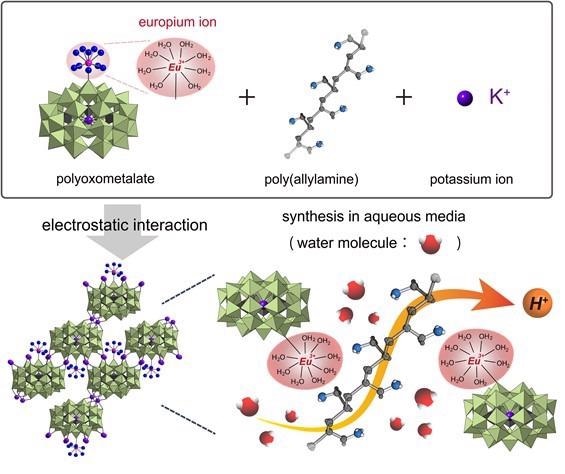 Ultra-high proton conduction via extended hydrogen-bonding network in polyoxometalate-based framework functionalized with lanthanide ion. Image Credit: Hiroshima University.
Ultra-high proton conduction via extended hydrogen-bonding network in polyoxometalate-based framework functionalized with lanthanide ion. Image Credit: Hiroshima University.
To expedite the development of the proton-based technology, an international research team has created a new hybrid material that successfully delivers protons at high humidity and temperatures—two crucial challenges faced in previous attempts.
The findings were published in American Chemical Society’s ACS Applied Materials & Interfaces journal on April 19th, 2021.
Headed by the University of Tokyo in Japan, the researchers targeted a material known as polyoxometalates (POMs), which they had already produced into a composite using compounds and another polymer to give structural stability.
POMs are attractive as building blocks for the design and synthesis of new materials with desirable properties and functions—they can efficiently transport protons, for example, but only at low temperatures and in low humidity. Unfortunately, a huge problem remained to be solved is that our composite decomposed at higher temperatures and humidity.
Masahiro Sadakane, Study Author and Professor, Graduate School of Advanced Science and Engineering, Hiroshima University
To overcome this issue, the team encapsulated positively charged ions in the internal cavities of the material to further modify the composite. To stabilize material conductivity, negatively charged ions, called anions, are balanced by positive ions, called cations.
The researchers settled on integrating a metallic element—called europium that remains solid at room temperatures—into the material. In particular, europium is attractive to water molecules and brings external oxygen into the material. Protons generally travel through the system by binding to oxygen. If there is more oxygen, the process will be more proton-conductive.
Our goal is to produce stable high proton-conductive materials. Through fine control of the components, we produced such a material.
Sayaka Uchida, Study Author and Associate Professor, Department of Basic Science, School of Arts and Sciences, The University of Tokyo
The material continued to show high proton conductivity at 50% humidity and at temperatures of 202.73 °F (368 °K). The team has planned to further boost the stability as well as proton conductivity.
We plan to increase the stability and proton conductivity so that this material can be used as an electrolyte in fuel cells, enhancing their performance. This work could provide guidance for the design of solid-state proton conductors.
Masahiro Sadakane, Study Author and Professor, Graduate School of Advanced Science and Engineering, Hiroshima University
Co-authors of the study include Tsukasa Iwano, Naoki Ogiwara, and Masanari Okuno from the Department of Basic Science, School of Arts and Sciences at The University of Tokyo; Kota Shitamatsu from the Department of Applied Chemistry, Graduate School of Advanced Science and Engineering at Hiroshima University.
Yuji Kikukawa from the Department of Chemistry, Graduate School of Natural Science and Technology at Kanazawa University; Satoru Ikemoto, Sora Shirai, and Satoshi Muratsugu from Graduate School of Science at Nagoya University; and Paul G. Waddell and R. John Errington from the Department of Chemistry, School of Natural & Environmental Sciences at Newcastle University are also the co-authors of the study.
The study was funded by the Japan Society for the Promotion of Science and the International Network on Polyoxometalate Science at Hiroshima University.
Journal Reference:
Iwano, T., et al. (2021) Ultrahigh Proton Conduction via Extended Hydrogen-Bonding Network in a Preyssler-Type Polyoxometalate-Based Framework Functionalized with a Lanthanide Ion. ACS Applied Materials & Interfaces. doi.org/10.1021/acsami.1c01752.