The University of Liverpool led a collaborative team of researchers to achieve the discovery of a new inorganic material that exhibits the lowest ever thermal conductivity.
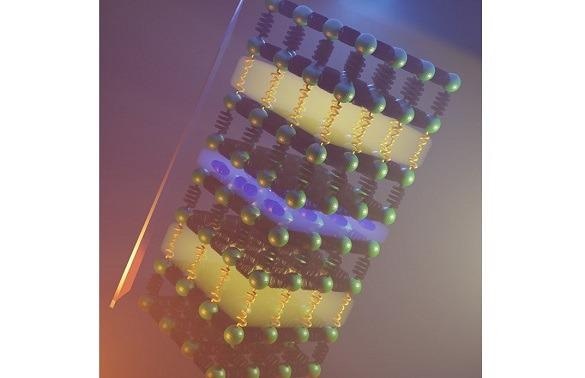 Using the right chemistry, it is possible to combine two different atomic arrangements (yellow and blue slabs) that provide mechanisms to slow down the motion of heat through a solid. This strategy gives the lowest thermal conductivity reported in an inorganic material. Image Credit: University of Liverpool.
Using the right chemistry, it is possible to combine two different atomic arrangements (yellow and blue slabs) that provide mechanisms to slow down the motion of heat through a solid. This strategy gives the lowest thermal conductivity reported in an inorganic material. Image Credit: University of Liverpool.
This breakthrough opens the door to developing new thermoelectric materials which would be critical for a sustainable society. The study has been published in the journal Science.
The research showcases an advance in controlling heat flow at the atomic scale, realized through materials design. The study also provides new basic insights into the management of energy. The new study will speed up the development of new materials for transforming waste heat to power and for the efficient use of fuels.
Headed by Professor Matt Rosseinsky from the University’s Department of Chemistry and Materials Innovation Factory and Dr. Jon Alaria from the Department of Physics and Stephenson Institute for Renewable Energy, the researchers engineered and produced the new material to blend two different arrangements of atoms identified to slow down the pace at which heat is transferred through the structure of a solid.
The team found the mechanisms that cause the decreased heat transport in each of the two arrangements through the measurement and modeling of the thermal conductivities of two different structures, where each one contained one of the needed arrangements.
The researchers found it challenging to combine these two mechanisms in a single material because it required precise control of how the atoms are arranged inside it. Researchers would anticipate an average of the physical properties of the two components to be achieved. The team opted for favorable chemical interfaces between each of these different atomic arrangements, and thus experimentally produced a material that combines both.
The newly developed material with two combined arrangements has a considerably lower thermal conductivity compared to either of the parent materials with just one arrangement. This unpredicted result reveals the synergic effect of the chemical control of atomic locations in the structure. This is the reason for the property of the whole structure to be superior to those of the individual parts.
If the thermal conductivity of steel is assumed as 1, that of a titanium bar is 0.1, that of water and a construction brick is 00.1, and the thermal conductivity of the new material is 0.001 and air is 0.0005.
The material we have discovered has the lowest thermal conductivity of any inorganic solid and is nearly as poor a conductor of heat as air itself. The implications of this discovery are significant, both for fundamental scientific understanding and for practical applications in thermoelectric devices that harvest waste heat and as thermal barrier coatings for more efficient gas turbines.
Matt Rosseinsky, Professor, Department of Chemistry and Materials Innovation Factory, University of Liverpool
“The exciting finding of this study is that it is possible to enhance the property of a material using complementary physics concepts and appropriate atomistic interfacing. Beyond heat transport, this strategy could be applied to other important fundamental physical properties such as magnetism and superconductivity, leading to lower energy computing and more efficient transport of electricity,” noted Dr. Jon Alaria.
Around 70% of all the energy produced across the globe is wasted as heat. Low thermal conductivity materials are necessary to decrease and harness this waste. The advent of new and more efficient thermoelectric materials enables the conversion of heat into electricity and is regarded as the primary source of clean energy.
This study was financially supported by the Engineering and Physical Science Research Council (EPSRC grant EP/N004884), the Leverhulme Trust, and the Royal Society.
Journal Reference:
Gibson, Q. D., et al. (2021) Low thermal conductivity in a modular inorganic material with bonding anisotropy and mismatch. Science. doi.org/10.1126/science.abh1619.