Reviewed by Alex SmithAug 31 2021
People are constantly on the lookout for cleaner alternatives, like hydrogen fuel, due to the existing global climate emergency and quickly receding energy resources.
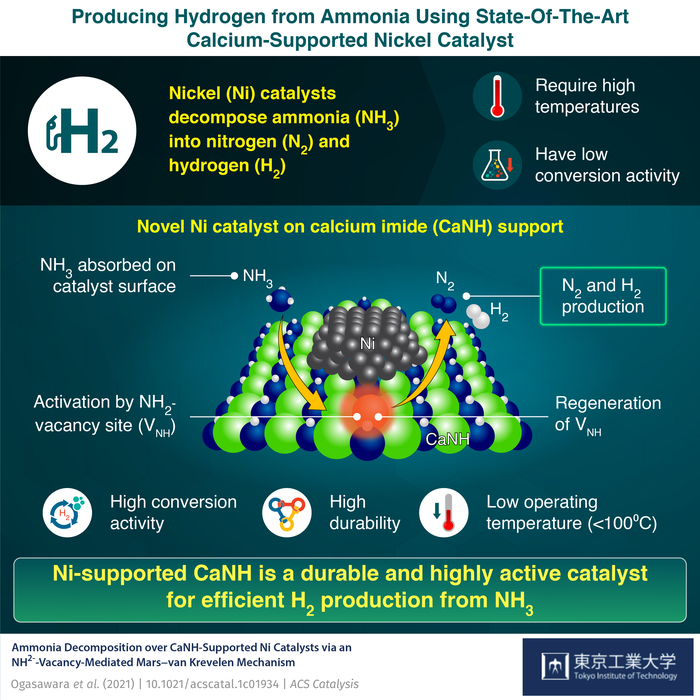
Image Credit: Tokyo Institute of Technology.
Hydrogen gas, when burnt in the presence of oxygen, creates large amounts of energy, without the noxious greenhouse gases, unlike fossil fuels. Disappointingly, the majority of the hydrogen fuel produced to date comes from fossil fuels or natural gas, which eventually raises its carbon footprint.
Ammonia (NH3), a carbon-neutral hydrogen compound, lately received significant attention due to its high energy density and high hydrogen storage capacity. It is possible to decompose ammonia to release hydrogen and nitrogen gases.
Ammonia can be readily stored, liquified, transported and transformed into hydrogen fuel based on the requirement. Yet, hydrogen production from ammonia is a slow reaction requiring very high energy demands. Metal catalysts are usually employed to speed up production, and they also help lower the overall energy consumption at the time of hydrogen production.
Current research works have discovered that Nickel (Ni) is a favorable catalyst for splitting ammonia. Ammonia gets adsorbed on the surface of Ni catalysts, resulting in the breakdown of bonds between nitrogen and hydrogen in ammonia and releasing them as individual gases. However, acquiring a better conversion of ammonia employing a Ni catalyst usually involves very high operating temperatures.
A group of scientists from Tokyo Tech headed by Associate Professor Masaaki Kitano portrayed a solution to overcome the problems encountered by Ni-based catalysts. The research has been published in the journal ACS Catalysis. The researchers fabricated a cutting-edge calcium imide (CaNH)-supported Ni catalyst that accomplished better ammonia conversion at low operating temperatures.
Our aim was to develop a highly active catalyst that would be energy efficient. Our addition of the metal imide to the catalyst system not only improved its catalytic activity but also helped us unravel the elusive working mechanism of such systems.
Masaaki Kitano, Associate Professor, Tokyo Institute of Technology
The researchers found that the presence of CaNH results in the development of NH2- vacancies (VNH) on the surface of the catalyst. These active species resulted in the enhanced catalytic performance of the Ni/CaNH at reaction temperatures 100 °C lower than those required for the functioning of Ni-based catalysts.
The scientists also created computational models and carried out isotope-labeling to comprehend the happenings on the catalyst’s surface. The calculations suggest a Mars–van Krevelen mechanism that involves adsorption of ammonia onto the CaNH surface, its activation at the NH2- vacancy sites, formation of nitrogen and hydrogen gas and ultimately the regeneration of vacancy sites promoted by Ni nanoparticles.
The extremely active and durable Ni/CaNH catalyst could be employed successfully for generating hydrogen gas from ammonia. Furthermore, the understanding of the mechanism of catalysis offered by this research can be employed to create a new generation of catalysts.
As the whole world is working together to build a sustainable future, our research is aimed at resolving the hiccups faced on our way to a cleaner hydrogen fuel economy.
Masaaki Kitano, Associate Professor, Tokyo Institute of Technology
The research offers new hope for the global low carbon-emission mission.
Journal Reference:
Ogasawara, K., et al. (2021) Ammonia Decomposition over CaNH-Supported Ni Catalysts via an NH2−-Vacancy-Mediated Mars–van Krevelen Mechanism. ACS Catalysis. doi.org/10.1021/acscatal.1c01934.