The faster development of nuclear power on a global scale has led to a continuous increase in the demand for uranium. Thus, huge amounts of uranium-containing wastewater are produced across the nuclear fuel cycle as well as at the time of spent fuel reprocessing.
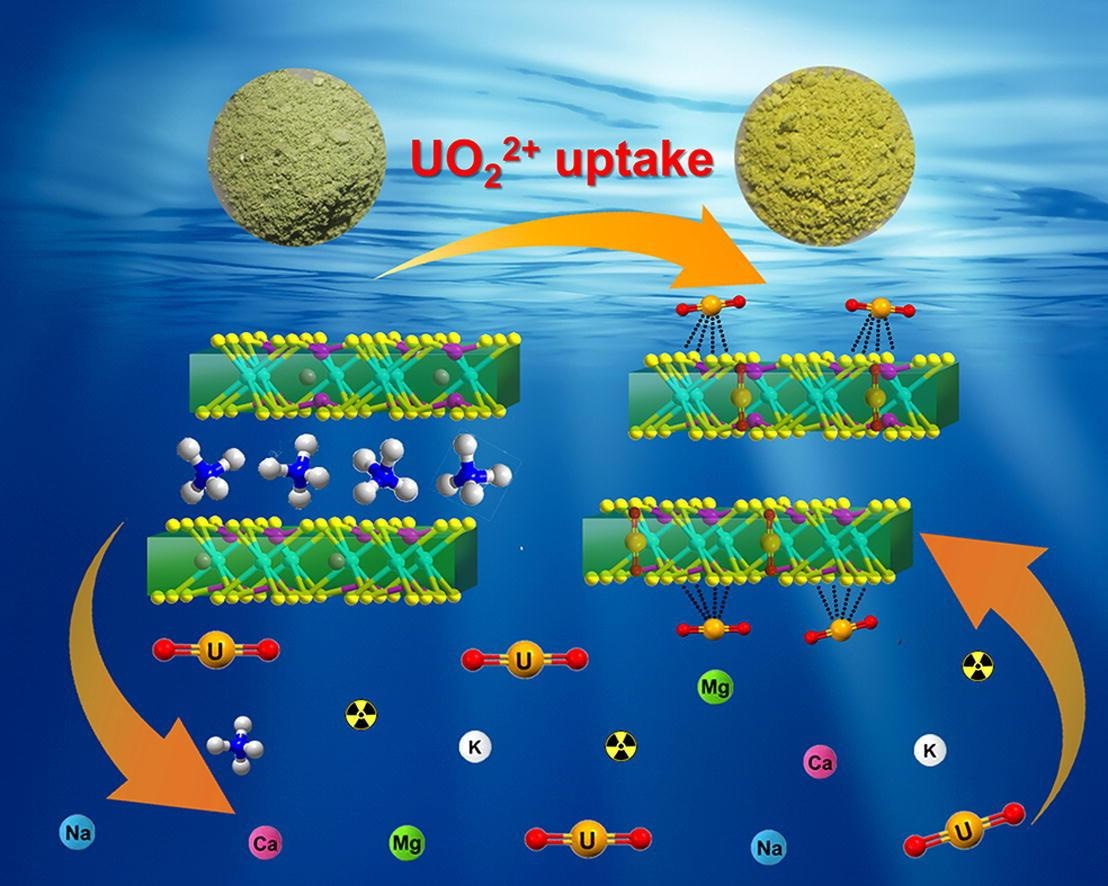 Schematic diagram of the layered MnPS3 intercalated with NH4+ (N-MPS) for the capture of U(VI). Image Credit: Professor Huang’s group.
Schematic diagram of the layered MnPS3 intercalated with NH4+ (N-MPS) for the capture of U(VI). Image Credit: Professor Huang’s group.
Uranium (U) is considered to be strongly carcinogenic and highly chemical toxic to the living system. Thus, it is essential to develop materials with the ability to capture U(VI) ions in an effective and selective manner.
In a study reported in the Chemical Engineering Journal, Professor Meiling Feng from Professor Xiaoying Huang’s research group at the Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences has come up with a layered manganese thiophosphate intercalated with NH4+, namely, (NH4)0.48Mn0.76PS3⋅H2O (N-MPS).
This could quickly and selectively isolate U(VI) from complicated solutions. This is the first instance of synthesizing an intercalation compound of manganese thiophosphite (MnPS3) for efficiently enriching U(VI).
The N-MPS was made by the scientists by immersing some amount of MnPS3 in 2 mol/L NH4Cl aqueous solutions and stirring it for 24 hours at room temperature. The synthesized N-MPS exhibits optimal pH durability (2.8 to 12.2) and β/γ-ray irradiation resistance ranging (from 100 to 200 KGy).
Furthermore, they discovered that N-MPS displayed an excellent capacity (quite greater compared to that of certain reported superior sulfide adsorbents), excellent selectivity towards U(VI) and fast kinetics.
The acquired distribution coefficient values in contaminated tap water and lake water are up to 2.23 × 104 mL/g. Notably, the adsorbed U(VI) can be eluted by a gentle and environmentally friendly technique to recover uranium and regenerate adsorbent for a minimum of five cycles.
The scientists showed that the adsorption mechanism of N-MPS for U(VI) is the synergy of ion exchange and surface adsorption. They achieved this with the help of numerous characterizations (XPS, Raman, EXAFS and Zeta potential) and batch adsorption experiments.
This research not only displays that intercalated metal sulfides possess good application potential for highly effective separation of U(VI) from wastewater but also sets the stage for developing new high-performance adsorbents through intercalation.
Journal Reference:
Zeng, X., et al. (2021) Efficient uptake of uranium(VI) by a layered manganese thiophosphite intercalated with NH4+. Chemical Engineering Journal. doi.org/10.1016/j.cej.2021.132474.