Researchers from ISTC, Italy, developed a titanium oxide-coated fabric, to be used as a photocatalyst agent to degrade the pollutants in water. Also, they analyzed various design options to achieve the best photodegradation efficiency and turnover frequency. This study is published in the journal Catalysts.

Study: Ceramized Fabrics and Their Integration in a Semi-Pilot Plant for the Photodegradation of Water Pollutants. Image Credit: voloshin311/Shutterstock.com
Advantages of Photocatalytic Water Treatment
The purification of water in a sustainable way is essential for the survivability of human beings in the future. Metal oxide semiconductor nanoparticles (NPs) such as TiO2 have garnered a lot of attention due to their photocatalytic behavior.
The photocatalytic activity of TiO2 is induced by UV light excitation with the consequent formation of electron-/hole+ pairs. One of the main applications for TiO2-based photocatalyst is in solar/visible light in areas without electricity or as a sustainable alternative to bio-hazardous and costly UV light.
Also, photocatalysts which are large, lightweight, and highly mechanically flexible that can be easily implemented in many different geometry water treatment reactors and are easily recoverable and regenerable are often preferable. Moreover, factors like low cost, eco-friendliness, high efficiency, and the breaking potential of a wide variety of organic molecules including viruses and chlorine-resistant organisms are important in the selection of photocatalysts.
About the Study
Researchers optimized TiO2-based photocatalytic fabrics, which were implemented in a semi-pilot plant reactor, investigated its quantum efficiency against Rhodamine B (RhB), when irradiated by both UV and visible light-emitting diode (LED) lights, and identified the optimized design options, catalyst selection, fabric properties, process parameters, and type of irradiation.
Acid TiO2 nano sol (TAC) with pH 1.5 was used to prepare two TiO2-based nanosuspensions viz. TACR and TiO2-SiO2 suspensions. The TACR was obtained by diluting TAC at the 3 wt. % concentration with deionized (DI) water and treated with anionic exchanger resin to increase the pH from 1.5 to 4.
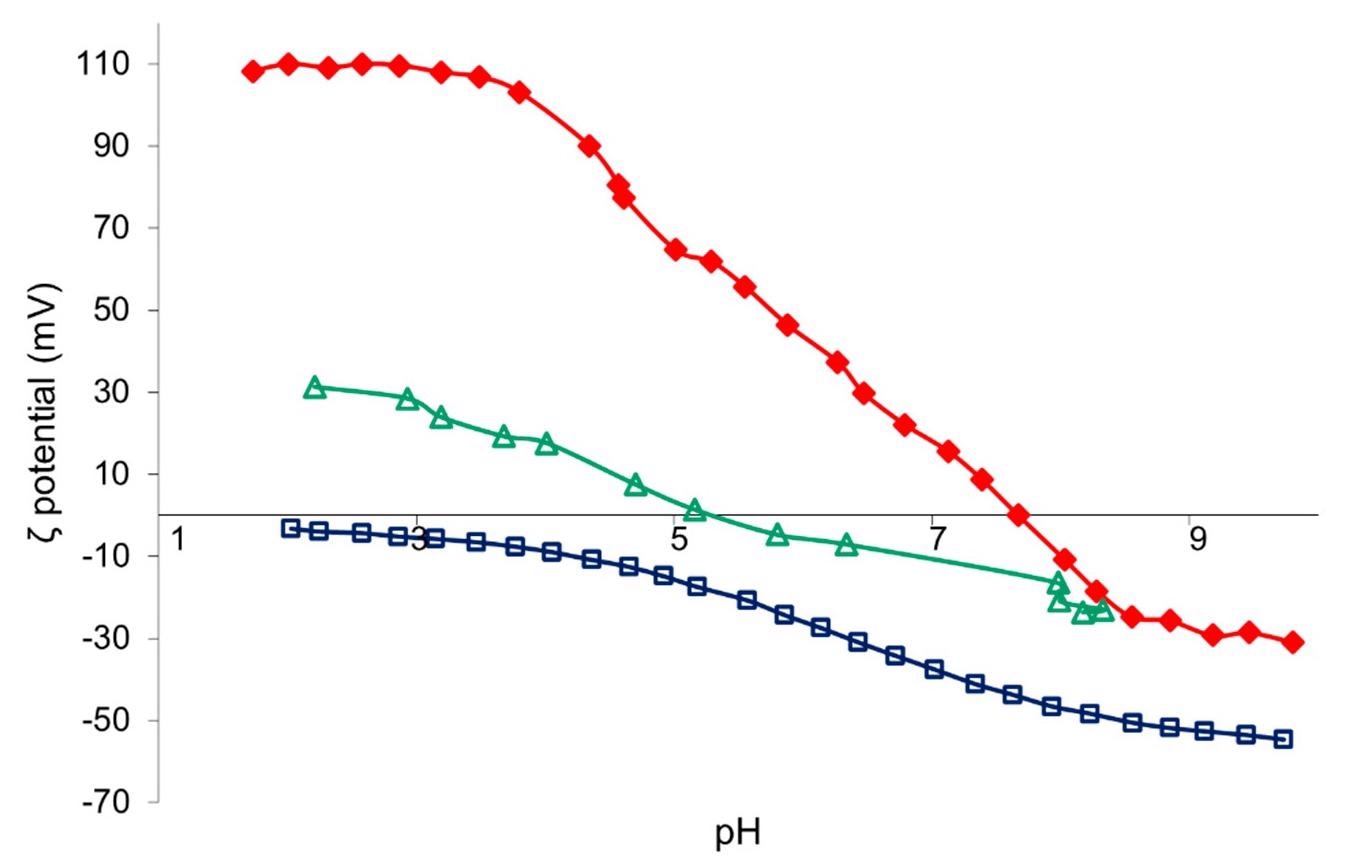
Z-potential vs. pH curves of TAC (red), SiO2 (blue), and TiO2:SiO2 (green) nanosuspension. Image Credit: Faccani, L., Catalysts
This increase in pH value is necessary to prevent fabric damage caused by high acidity, also the residue formed by the synthesis of original TiO2 can reduce the photocatalytic activity. The TiO2-SiO2 3 wt. % mixture was prepared by the hetero-coagulation method. SiO2 nano sol 40 wt. % was diluted at the concentration of 3 wt. % with DI water and treated with ion acidic cationic exchanger resin to decrease the pH from 10 to 4 (SiO2-R).
TiO2 suspension (TACR) was dropped into SiO2-R suspension. The TiO2-SiO2 sample was obtained through electrostatic interaction between negatively charged silica nanoparticles and positively charged titania nanoparticles.
The coated fabrics were formed through the dip-pad-curing process. The fabric was dipped in a TiO2-based suspension followed by squeezing, drying, and curing.
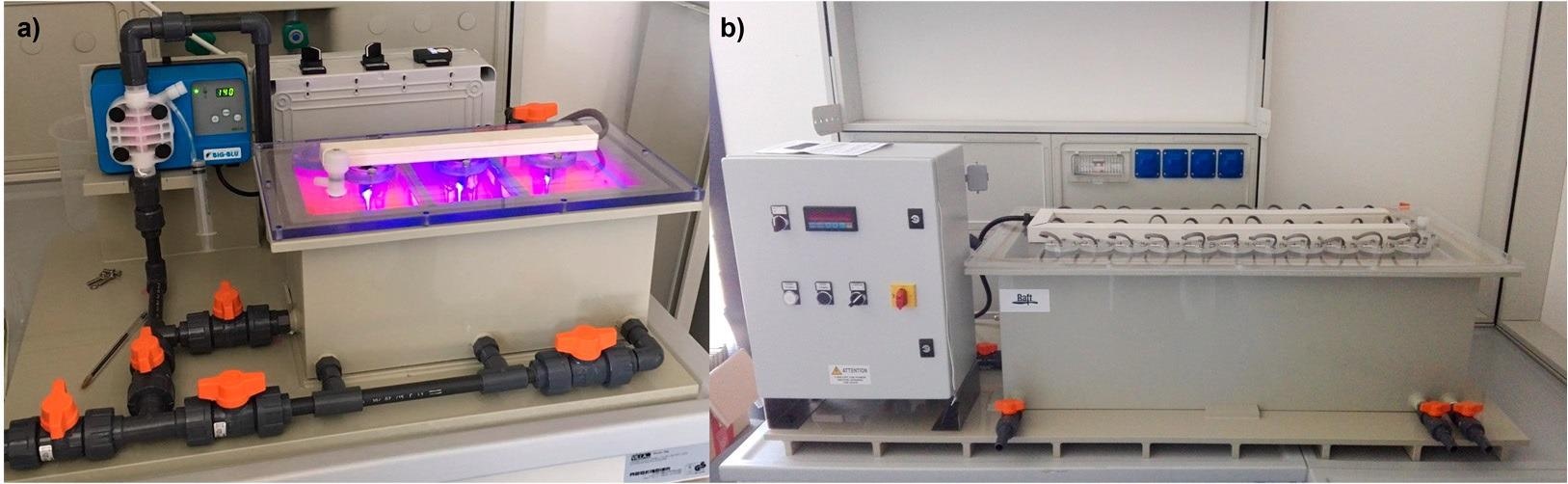
(a) LED-based semi-pilot photocatalytic reactor tested (6L), used in this study; (b) Up-scaled reactor (100L) built upon the best design options in the present study. Image Credit: Faccani, L., Catalysts
Observations
The hydrodynamic diameter, Z-potential, and pH values of pure materials and TiO2-based nanosuspensions were measured. Hydrodynamic diameter is found to be increased slightly and the presence of zeta potential is observed in samples obtained after resin treatment of TAC and SiO2 namely, TACR and SiO2-R owing to a decrease in the colloidal stability coupled and pH change.
However, a large increase in the hydrodynamic diameter in the TiO2-SiO2 sample is due to the steric hindrance of SiO2 hetero-coagulation on the TiO2 surface. The contact angle measurements show all samples with hydrophilic properties and no significant differences between untreated and treated fabrics.
The RhB photodegradation of the two photocatalysts over time shows a progressive decrease in the absorbance of the RhB peak at 554 nm. Moreover, both photocatalysts demonstrated a blue shift of the maximum wavelength.
This is associated with a de-ethylation of RhB molecules, which is in agreement with the hypothesized RhB degradation mechanism, in the presence of selected photocatalysts. Moreover, they found that the blue-shift phenomenon due to the RhB de-ethylation is more profound under visible light than UV light, which is because the UV radiation directly excites the TiO2, whereas under visible light the RhB is absorbed on the surface of the photocatalyst to subsequently produce the active oxygen molecules.
The temperature dependency study revealed that increasing temperature promotes phenomena such as the desorption of adsorbed reactants and the rate of recombination of photogenerated electron-hole pairs, which are detrimental for photocatalysis. From all angles, the room temperature is found to be the best-compromised working temperature with matching environmental and economic requirements.
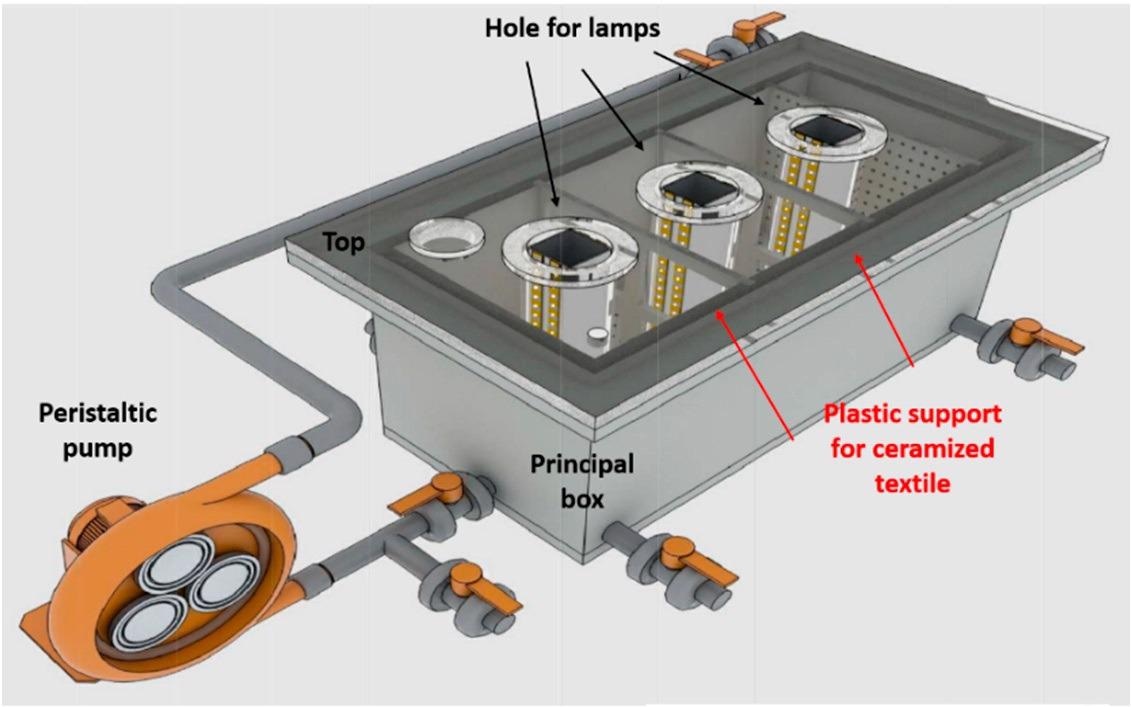
Schematized representation of a 6 L semi-pilot plant. Image Credit: Faccani, L., Catalysts
Conclusions
The authors immobilized TiO2-based nanoparticles as fabric coatings and obtained low-cost and highly flexible photocatalysts that can be easily implemented in many geometry water treatment reactors. They used a multi-variables optimization approach to find photoreactor operating parameters that affected the photocatalytic performance the most and identify the best design option in terms of safety and sustainability.
They found that a 100% biodegradable cotton fabric irradiated by visible LED is the best candidate on all aspects and it showed higher efficiency than all the other samples that were irradiated by both cost-effective visible light and UV light sources. The favorable results encouraged the scale-up of the process from the 6 L semi-pilot plant up to the 100 L pilot plant.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Faccani, L., Ortelli, S., Blosi, M., Costa, A.L, Ceramized Fabrics and Their Integration in a Semi-Pilot Plant for the Photodegradation of Water Pollutants. Catalysts 2021, 11, 1418. https://www.mdpi.com/2073-4344/11/11/1418