A new research paper posted to the chemRxiv* preprint server focuses on the complex process of narcissistic self-sorting in supramolecular chains that can aid in the construction of substances with more complicated topologies.

Study: Chemically Fueled Self-sorted Hydrogels. Image Credit: Alexa_Space/Shutterstock.com
The materials offer intriguing mechanical characteristics, such as increased or decreased rigidity and complex multistep gelation kinetics. Furthermore, the authors demonstrate supramolecular templating, in which pre-existing supramolecular fibers initially function as precursors for the formation of a subsequent gelator, after which they may be selectively deleted.
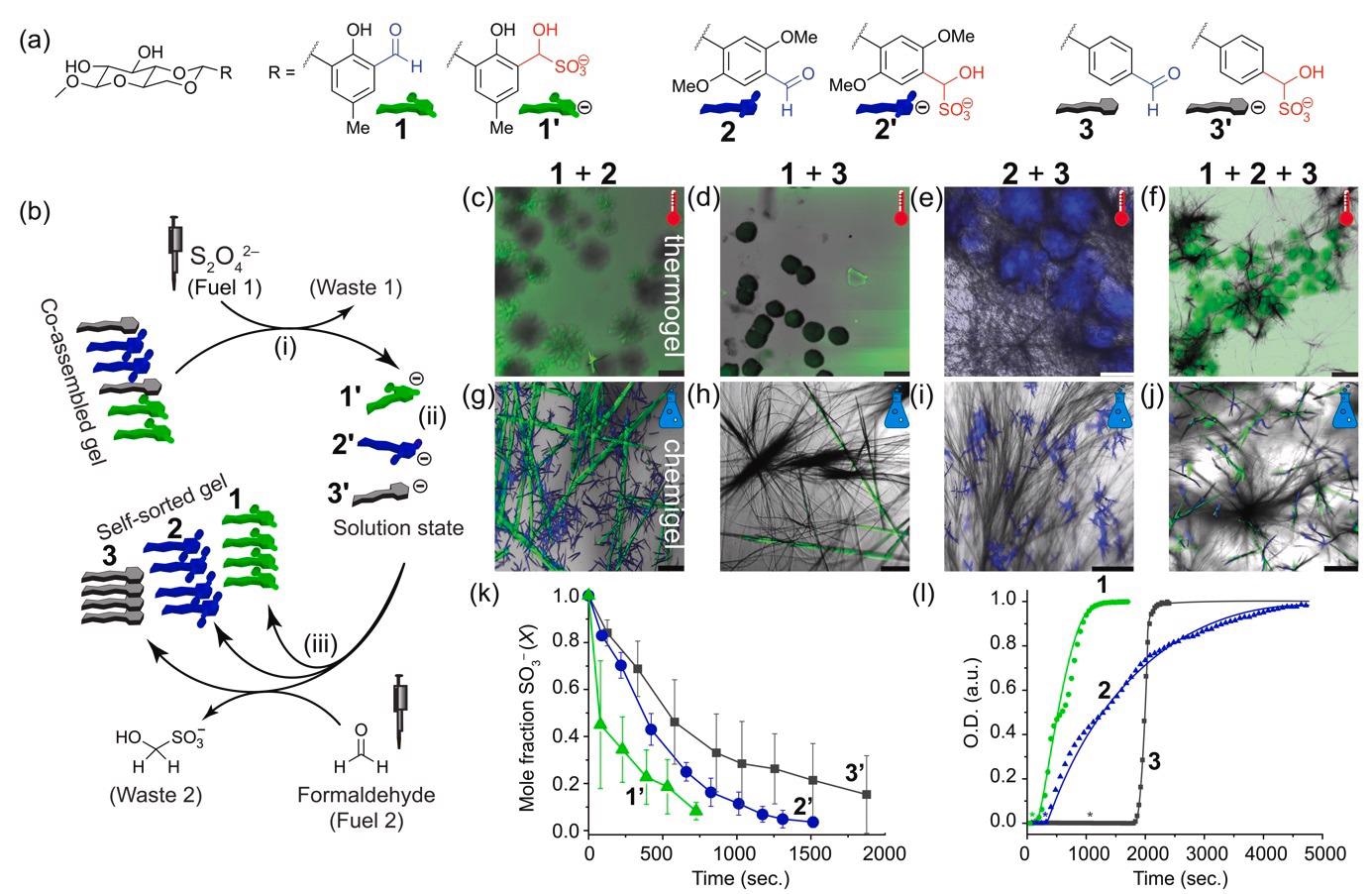
Self-sorting achieved by a chemical fuel. (a) Chemical structures of the used gelators, and their hydroxysulfonate analogs (numbers with prime indication). (b) Scheme starting from a co-assembled thermogel that is disassembled by fuel 1 (i), resulting in a solution state (ii). After addition of fuel 2 (iii) a self-sorted gel is formed. (c–j) Confocal images of thermally annealed (c–f), or chemically fueled (g–j) assemblies. (k) Kinetics of consumption of 1’, 2’, and 3’ (21.6 mM) after HCHO (~44 eq.) addition from 1H-NMR. (l) Self-assembly kinetics from turbidity measurements at 500 nm using UV-Vis spectroscopy (see Figure S9 for detailed concentration dependent measurements). Assemblies of 1, 2, and 3 (21.6 mM) from 1’, 2’, and 3’ upon addition of HCHO (47 eq.). Lines to guide the eye. Lag phases are indicated with asterisks. Image Credit: Singh, N. et al., ChemRxiv
Importance and Utilization of Hydrogels
Hydrogels captivate material engineers and biological scientists, and significant progress has been achieved in their synthesis and uses.
Hydrogels are frequently employed in polarization, biomedical separation processes, proteomic, chromatography, tissue regeneration, and other fields. They are popular as an efficient absorbent material in disposable diapers, core filtration material for water treatment, and isolation materials for centrifugation and electrophoresis in foods and medications. They are also useful for sustained drug release and purification of dilute macromolecule formulations.
Limitations of Hydrogels
Despite the fact that hydrogels are used for a variety of reasons, there are a number of drawbacks to them. The reaction time of hydrogels is size-dependent, which is due to the sluggish dispersion of molecules of water. The reaction time of hydrogels with diameters of several centimeters might be many hours. However, porous hydrogels can be used to overcome this problem.
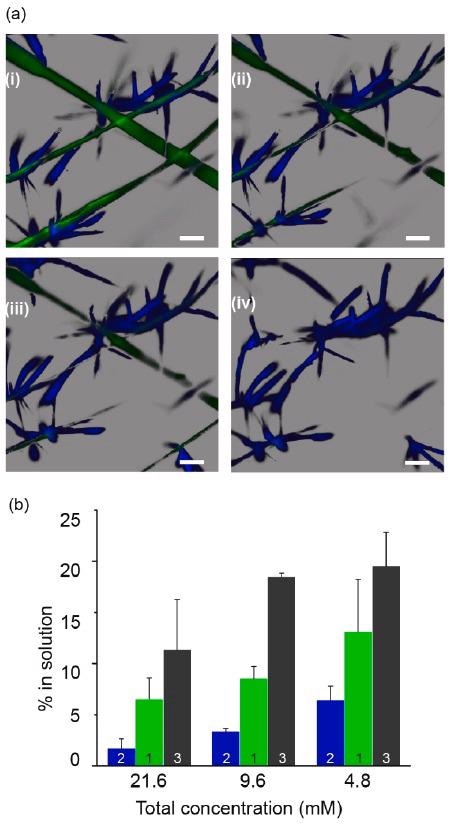
Solubility differences allow for selective supramolecular template removal. (a) Confocal microscopy timelapse images showing selective removal of green scaffold (1) by slow addition of sodium dithionite DT while keeping the blue fluorescent assemblies of 2 intact. Scale bars: 50 μm, time interval between images i–iv: 20 min. (b) Percentage of soluble molecules as a function of total solution concentration of individual assemblies of 1–3, determined by 1H NMR in comparison with a soluble internal standard (hydroquinone). Image Credit: Singh, N. et al., ChemRxiv
Introduction to Narcissistic Self-Healing
The dynamic blending of constituents, solution switching, chiral detection, electrostatic interactions, pH modification, and supramolecular catalyst supports have all been used to create narcissistically self-sorted hydrogels. The most frequent method is a temperature annealing treatment, in which varied gelation temperatures of the molecules allow them to self-assemble progressively. A second method employs a gradual pH adjustment to produce two-component self-sorted hydrogels.
Result Findings
It was shown in the latest research that a chemically fueled substituent functional group change, such as aldehyde–to–hydroxy sulfonate (and back), can result in excellent control over self-sorting, allowing access to well-structured three-component hydrogels. The initial step was the utilization of a controlled cooling approach. Three different hydrogel substances were synthesized.
Microscopy showed that thermogel 1 had green fluorescent particles, while thermogel 2 and 3 displayed blue spherulites and non-fluorescent fibers. It was found that it is difficult to anticipate narcissistic self-sorting in architecturally identical molecules at an equilibrium point. Excluding the mixture of type 2+3 thermogel, most multiphase thermogels were unable to self-sort. Instead, co-assembly was favored, resulting in the loss of fibrillar shape and, in some cases, gel-forming capabilities.
The next analysis was of the chemically fuelled gels or ‘chemigels’. When contrasted to the thermogel, type 1 produced green particle emitters fibers. Compound 2 was still producing spherulites, although they are 20 times smaller (at 20–30 m) than in the thermally treated instance.
Thermally, the fibers were randomly scattered in space, but chemically, they develop more from specified nucleation centers into fractal-like formations owing to secondary nucleation. Time-dependent kinetics was used to assess the rates at which the different hydroxy sulfonates reverted back to their corresponding aldehydes.
The mechanical testing revealed that due to the lengthy wavy threads seen in supramolecular hydrogels, hydrogels of 3 exhibited the best mechanical behavior, creating the toughest of the three substances. The combination of the first and third hydrogel had a very high mechanical strength than the individual. It is due to the lengthy extended overlapping fiber network that fills the vacant spaces creating a tight densely packed molecular structure.
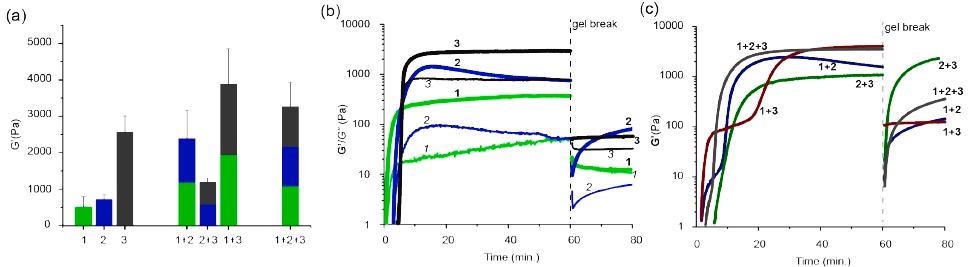
Mechanical characterization of chemically fueled multicomponent gels. (a) Storage moduli (G’) of one, two, and three component chemically-fueled self-sorted hydrogels. (b) Time-evolution of hydrogels after adding HCHO to hydroxysulfonate 1’, 2’, or 3’ solutions. Gel breaking (dashed vertical line) was performed by applying a high shear rate (1000 s–1) at 60 min. (c) The same as panel b but for two, and three component mixtures. Image Credit: Singh, N. et al., ChemRxiv
Limitations of the Study
Man-made chemically fueled systems have already demonstrated exciting features including vibrations, vibrant encapsulation, and transient assembly, but they have not been used to manage the structure of complex systems. Although ATP-powered transiently self-sorted micelles employing DNA building blocks have been shown, a corresponding technique in chemically fueled synthetic materials has been absent.
In conclusion, the research successfully proved how molecular fuels may be employed to produce multicomponent self-sorted hydrogels. Subtle changes in the chemical composition of the hydrogelators influenced both their responsiveness to chemical fuels and their proclivity to self-assemble. As a consequence, complicated self-sorted materials comprised of molecules might be created using standard methods. The innovative method even allows for the usage of supramolecular frameworks. In other words, a first assembly directs the second, after which the first can selectively remove itself.
*Important Notice
ChemRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive or treated as established information.
References
Singh, N., Acosta, A. L., Formon, G. J. & Hermans, T. M., 2021. Chemically Fueled Self-sorted Hydrogels. ChemRxiv. Available at: https://chemrxiv.org/engage/chemrxiv/article-details/61a49fe4dff1cc45eb364d55
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.