With climate change persisting, a growing number of scientists are concentrating on enhancing electric vehicles (EVs) to make them a more appealing alternative to standard gas cars. The battery enhancement of EVs is a significant issue to attract additional drives.
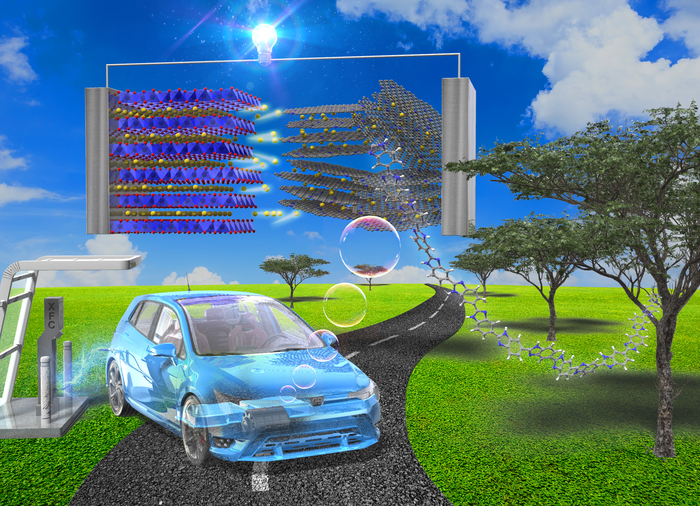 Poly (benzimidazole), the precursor for the proposed anode material, can be derived from biological processes and processed easily to create fast-charging lithium-ion batteries. Their adoption of electric vehicles will make them more attractive to consumers than conventional cars, leading to cleaner environments and reduced CO2 emissions. (Image Credit: Noriyoshi Matsumi from JAIST).
Poly (benzimidazole), the precursor for the proposed anode material, can be derived from biological processes and processed easily to create fast-charging lithium-ion batteries. Their adoption of electric vehicles will make them more attractive to consumers than conventional cars, leading to cleaner environments and reduced CO2 emissions. (Image Credit: Noriyoshi Matsumi from JAIST).
Besides autonomy, safety and durability, the majority of people want rapid charging. Presently, it takes 40-minutes with advanced EVs while gas cars can be ‘recharged’ within 5 minutes. The charging time has to be less than 15 minutes to be a feasible option.
Predictably, lithium-ion batteries (LIBs), which are used universally with portable electronic gadgets, have been accepted as an option in the field of EVs, and new approaches are continually being pursued to enhance their performance.
One method to cut the charging time of LIBs is to boost the diffusion rate of lithium ions, which consecutively can be achieved by increasing the interlayer distance in the carbon-based materials found in the battery’s anode.
While this has been accomplished with some success by adding nitrogen impurities (technically called ‘nitrogen doping’), there is no technique easily available to regulate interlayer distance or to concentrate the doping element.
Against this background, a group of researchers from the Japan Advanced Institute of Science and Technology (JAIST) recently formulated a method for anode fabrication that could pave the way to the very fast charging of LIBs.
The team, guided by Prof. Noriyoshi Matsumi, includes Prof. Tatsuo Kaneko, Senior Lecturer Rajashekar Badam, JAIST Technical Specialist Koichi Higashimine, JAIST Research Fellow Yueying Peng, and JAIST student Kottisa Sumala Patnaik, and their findings have been published online on November 24th, 2021 in the journal Chemical Communications.
Their method constitutes a comparatively simple, environmentally sound and very efficient process to create a carbon-based anode with a very high nitrogen quantity. The precursor material for the anode is poly (benzimidazole), a bio-based polymer that can be fabricated from raw materials of natural origin.
The researchers calcinated this thermally stable material at 800 °C and succeeded in preparing a carbon anode with a record-setting nitrogen content of 17% in weight. They confirmed the fruitful synthesis of this material and examined its composition and structural properties using a range of techniques, including Raman spectroscopy, scanning electron tunneling microscopy and x-ray photoelectron spectroscopy.
To verify their anode’s performance and compare it with the more standard graphite, the team constructed full-cells and half-cells and carried out charge-discharge experiments. The results were very favorable, as the anticipated anode material proved ideal for rapid charging, owing to its superior lithium-ion kinetics.
Furthermore, durability tests revealed that the batteries with the anticipated anode material retained approximately 90% of their original capacity even after 3,000 charge-discharge cycles at high rates, which is significantly beyond the capacity held by graphite-based cells.
Professor Matsumi was happy with the results.
The extremely fast charging rate with the anode material we prepared could make it suitable for use in EVs. Much shorter charging times will hopefully attract consumers to choose EVs rather than gasoline-based vehicles, ultimately leading to cleaner environments in every major city across the world.
Professor Noriyoshi Matsumi, Study Lead, Japan Advanced Institute of Science and Technology
Another prominent advantage of the anticipated anode material is the use of a bio-based polymer in its fabrication. As a low-carbon technology, the material certainly leads to a synergistic effect that decreases CO2 emissions further.
The use of our approach will advance the study of structure–property relationships in anode materials with rapid charge–discharge capabilities.
Professor Noriyoshi Matsumi, Study Lead, Japan Advanced Institute of Science and Technology
Alterations to the structure of the polymer precursor could result in even better performance, which might be applicable for the batteries not only of EVs but also of handy electronics. Finally, the creation of highly durable batteries will cut the worldwide consumption of rare metals, which are non-renewable resources.
Future progress in this field will make way for the extensive adoption of electric cars and other sustainable technologies.
Journal Reference:
Patnaik, K. S., et al. (2021) Extremely Fast Charging Lithium-ion Battery Using Bio-Based Polymer-Derived Heavily Nitrogen Doped Carbon. Chemical Communications. doi.org/10.1039/D1CC04931C.