The latest study published in the journal Sustainability reflects light over the influence of four distinct varieties of brine at varying salt concentrations (5 to 25 wt.%) on carbon dioxide storage.
Study: Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers. Image Credit: Dmitry Kovalchuk/Shutterstock.com
It should be kept in mind that the salt deposition during CO2 sequestration in saltwater aquifers can have a significant impact on injectivity.
Carbon Emissions and Importance of their Reduction
Owing to a large rise in the emission of greenhouse gases, the usage of fossil fuels has resulted in severe environmental problems such as the increase in global warming. Carbon dioxide (CO2) is the primary cause of global warming since its quantity in the environment has grown dramatically over the last few decades.
Carbon Capture and Storage (CCS) technology offer the ability to minimize anthropogenic CO2 emissions while securely sequestering them in geological formations such as drained petroleum reservoirs or deep saline deposits. This technique has the potential to reduce CO2 emissions by up to 17% by 2050. As a result, tectonic absorption, or the infusion of CO2 into exhausted petroleum resources, underground coal seams, and groundwater aquifers, is gaining popularity.
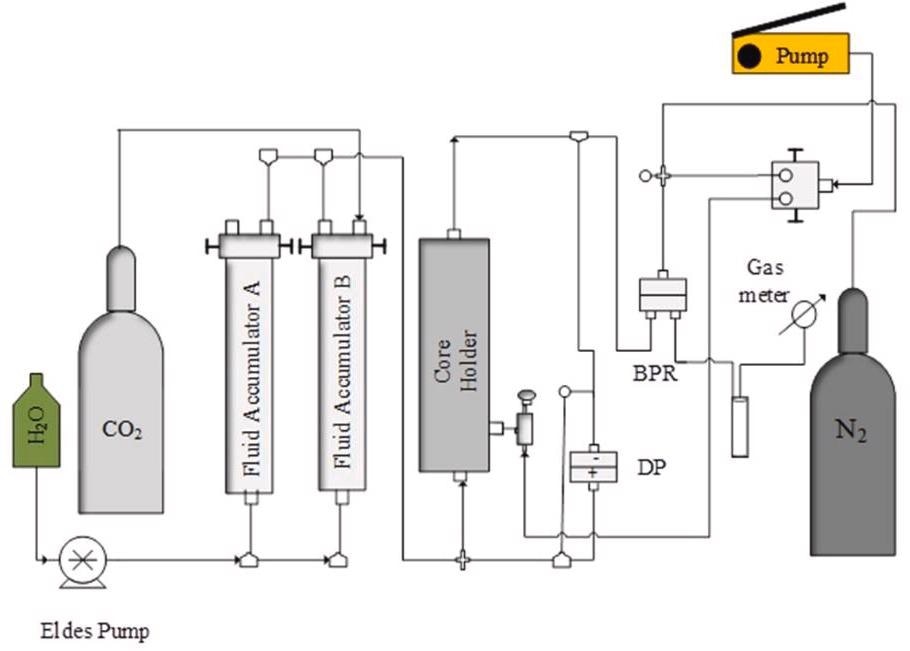
Schematic diagram of the core flooding experimental setup showing the path of the pressure decay test. Image Credit: Edem DE et al., Sustainability
Importance of Brine Solutions for Carbon Storage
Owing to their greater capacity, capturing method, and widespread availability, CO2 injection in deep saline aquifers (sequestration) has the highest potential for capturing CO2. The evaporation of development brine is one of the key issues facing carbon capture in aquifers.
This vaporization causes a salt deposition reaction, which may ultimately have a negative influence on injectivity, preventing CO2 accumulation. The infusion of a substantial volume of CO2 into the formation water causes salt deposition in deep saline aquifers, resulting in water evaporation and a rise in the molar percentage of water in the CO2 stream.
The process of evaporation is the core reason for the increase in levels of solutes in the brine continuously. The amount of salt surpasses its absorption to some degree in the groundwater aquifers' heat capacities, leading to the deposition of additional salts from the aqueous solution. This process, known as salting out, changes the pore size distribution and flowability of the deposit.
Factors Affecting Salt Precipitation
The concentration of salt, the pace of dehydrating, the dispersion of deposited salt in the crevices of the material, and the rock properties are all elements that influence salt deposition. Other characteristics relevant in emulsification include the size and charges of the ions, the temperatures of the saltwater, the amount of salt, and the solvents' constant dissipation factor.
CO2 dissolution in a saturated zone aquifer is pressure-sensitive, and pressure rises with lowering the temperature, leading to an increase in CO2 absorption. Furthermore, because absorption is directly proportional to the quantity of salt in each element, an increment in dissolved salts leads to emulsification. According to the findings, KCl has a smaller salting-out impact than NaCl and CaCl2. Furthermore, MgCl2 has a greater salting-out impact than NaCl or KCl, which have a comparable effect.
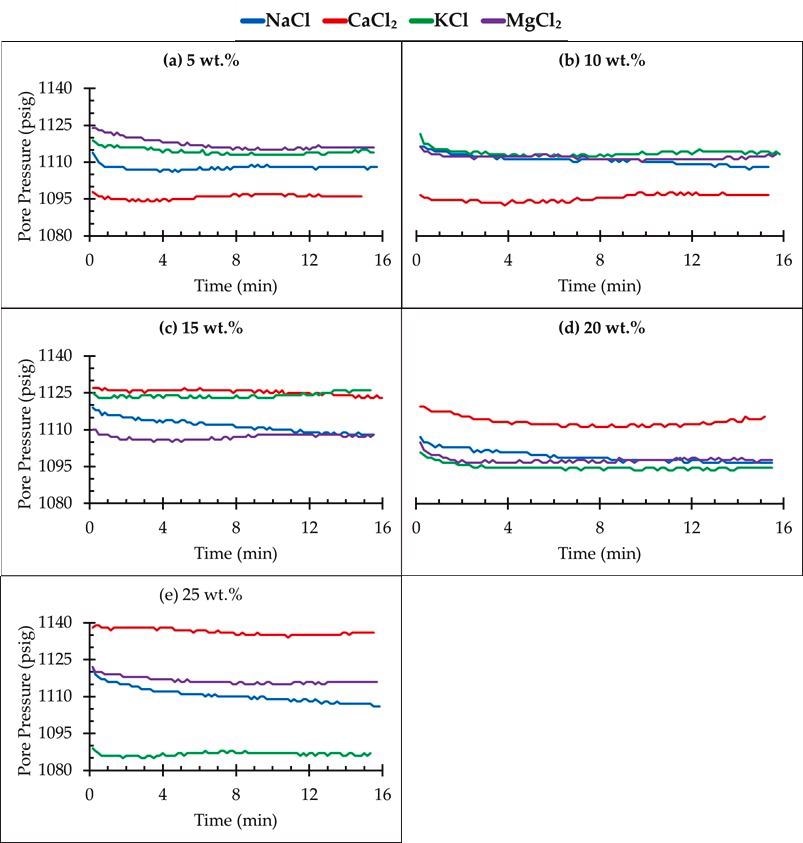
Pressure decay test for different salt concentrations. Image Credit: Edem DE et al., Sustainability
The most recent study investigates a broad array of brine contents for various salt varieties to obtain the optimum saline percentage bunch of alternative salt kinds for successful CO2 sequestration in a groundwater aquifers sand reservoir. Furthermore, saline aquifers inside sandstones have been sought.
Research Methods
Helium and 99 percent pure liquid carbon dioxide were utilized. Four different salts (NaCl, KCl, CaCl2, and MgCl2) were used to create brine samples. NaCl is the most common brine substance found in saline aquifers, accounting for 70 to 90 percent of the total. The Core Flooding technique was employed. The fundamental physical properties of the core sample were determined after drying it in an oven at 75 C for 24 hours to eliminate any trace of solvent and moisture left over from the Soxhlet extraction cleaning.
Research Findings
MgCl2 brine had the earliest CO2 breakout at a 15 wt. percent concentration, trailed by NaCl, KCl, and CaCl2. This meant that a significant quantity of CO2 was incorporated in the CaCl2 saline, which had a delayed breakout time when compared to the other brines, with all experiments performed under identical circumstances and with extremely excellent reproducibility. In all of the flooding situations,
NaCl exhibited the greatest decay rates, suggesting that NaCl solutions had the maximum CO2 solubility. The results also showed that CO2 was more soluble in monovalent salts than in divalent salts (CaCl2 and MgCl2). Furthermore, the disintegration rate was found to be maximum in sand loaded with NaCl brine.
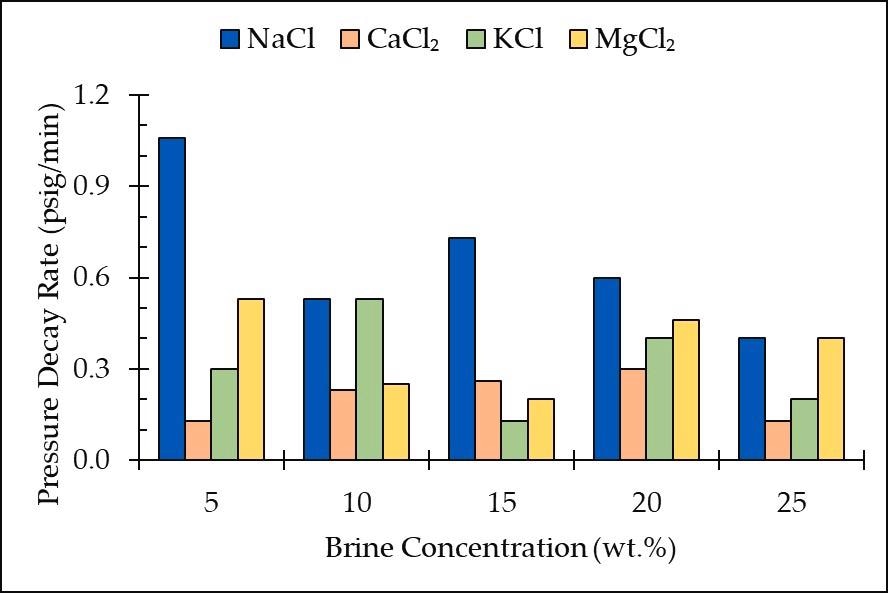
Pressure decay rate vs. brine concentration for all the salt types and concentrations. Image Credit: Edem DE et al., Sustainability
In short, the research showed that the best concentration range for carbon storage in salty groundwater aquifers is 10 wt. percent to 20 wt. percent. However, experiments on brines containing diverse concentrations of different salt should be performed to find moderating salt forms in terms of storage effectiveness.
Further Reading
Edem DE, Abba MK, Nourian A, Babaie M, Naeem Z. Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers. Sustainability. 2022. 14(2). 986. Available at: https://www.mdpi.com/2071-1050/14/2/986
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.