Amongst abundant and sustainable natural materials, sugar can be used to produce polymers, and a new study published in the Journal of the American Chemical Society has focused on investigating the stereochemical properties of sugar to produce new classes of commercially viable polymers.

Study: Sugar-Based Polymers with Stereochemistry-Dependent Degradability and Mechanical Properties. Image Credit: Pressmaster/Shutterstock.com
The Influence of Stereochemistry on Synthetic Polymers
Natural compounds display unique structures and stereoisomerism that can inform research into the creation of mechanically robust materials which display behavior defined by stereochemistry.
The influence of stereochemistry on the backbone of synthetic polymers is well-recognized. For example, the chain-packing behavior of polypropylene is affected by the stereochemical relationship between neighboring methyl groups on the polymer’s backbone. By increasing the stereoregularity of neighboring units, the efficiency of polymer chain packing can be improved, leading to increased crystallinity, which in turn improves the polymer’s toughness and strength.
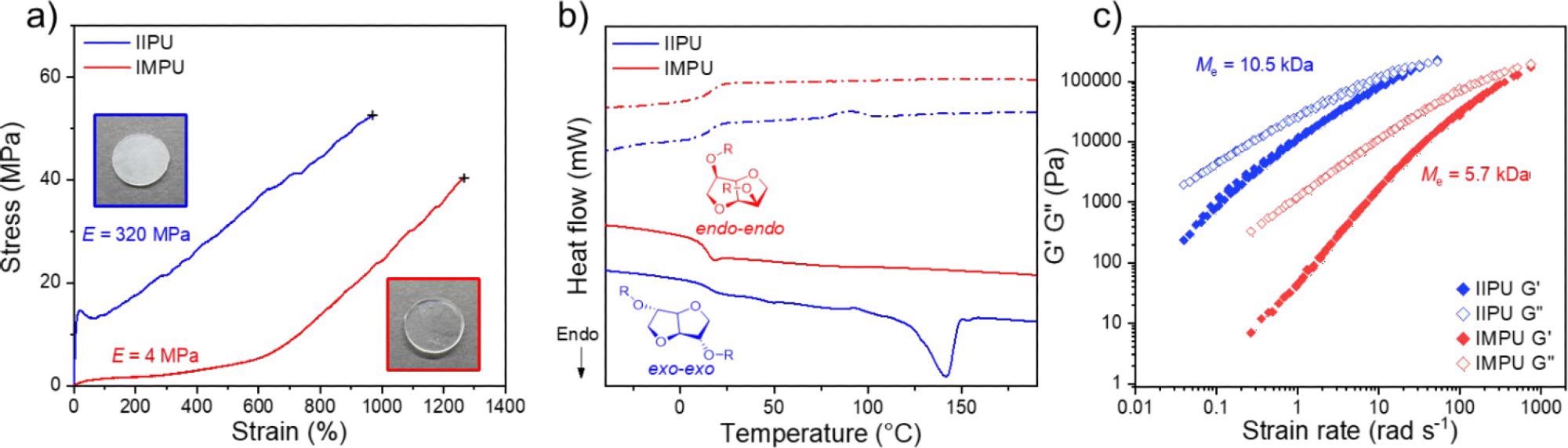
Thermomechanical analysis of IIPU and IMPU. (a) Representative stress vs strain curves obtained at 10 mm·min–1, 22 °C for annealed IIPU and IMPU with Young’s modulus (E) displayed (inset: photographs of pressed films of IIPU and IMPU). (b) DSC thermograms of the first heating and cooling cycle for the annealed (3 days, 22 °C) IIPU and IMPU (solid line = heating scan and dashed line = cooling scan). (c) Time–temperature superposition of rheological frequency sweeps of IIPU and IMPU overlaid at 130 °C. Chain entanglement molecular weight (Me) of IIPU and IMPU is displayed. Image Credit: Stubbs, C.J et al., Journal of the American Chemical Society
Research into important bioplastics like polylactic acid has also turned to stereochemistry to improve the thermomechanical properties of materials. However, in both polylactic acid and polypropylene, plastic deformation under loading is retained irrespective of stereochemistry. This is unlike the fundamental differences in mechanical behavior observed in natural rubber, which displays elastic behavior, and gutta-percha, which is plastic.
Manufacturing Functional Polymers from Sugar
Rigid-ring bicyclic ethers, which are produced by the dehydrative cyclization of sugar hexitols, possess unique structural features and can impart geometric isomerism, which has made them attractive candidates for use as monomers. The most common among these compounds isosorbide, which is derived from glucose. However, isoidide and isomannide, which are stereoisomers of isosorbide, have received little attention in research.
Studies into these sugar-derived stereoisomers have reported polymers synthesized with them, but do not possess detailed mechanical and/or thermal characterization. Thus, the influence of stereochemistry has not been fully elucidated in historic and recent research.
The structure of the isohexides is bowl-shaped. Polymerization of isodorbide-based monomers typically produces regioirregular polymers, with a few exceptions produced using ring-opening polymerization or polymerizing unsymmetrical isosorbide-based monomers. This makes producing functional polymers with desired mechanical effects difficult.
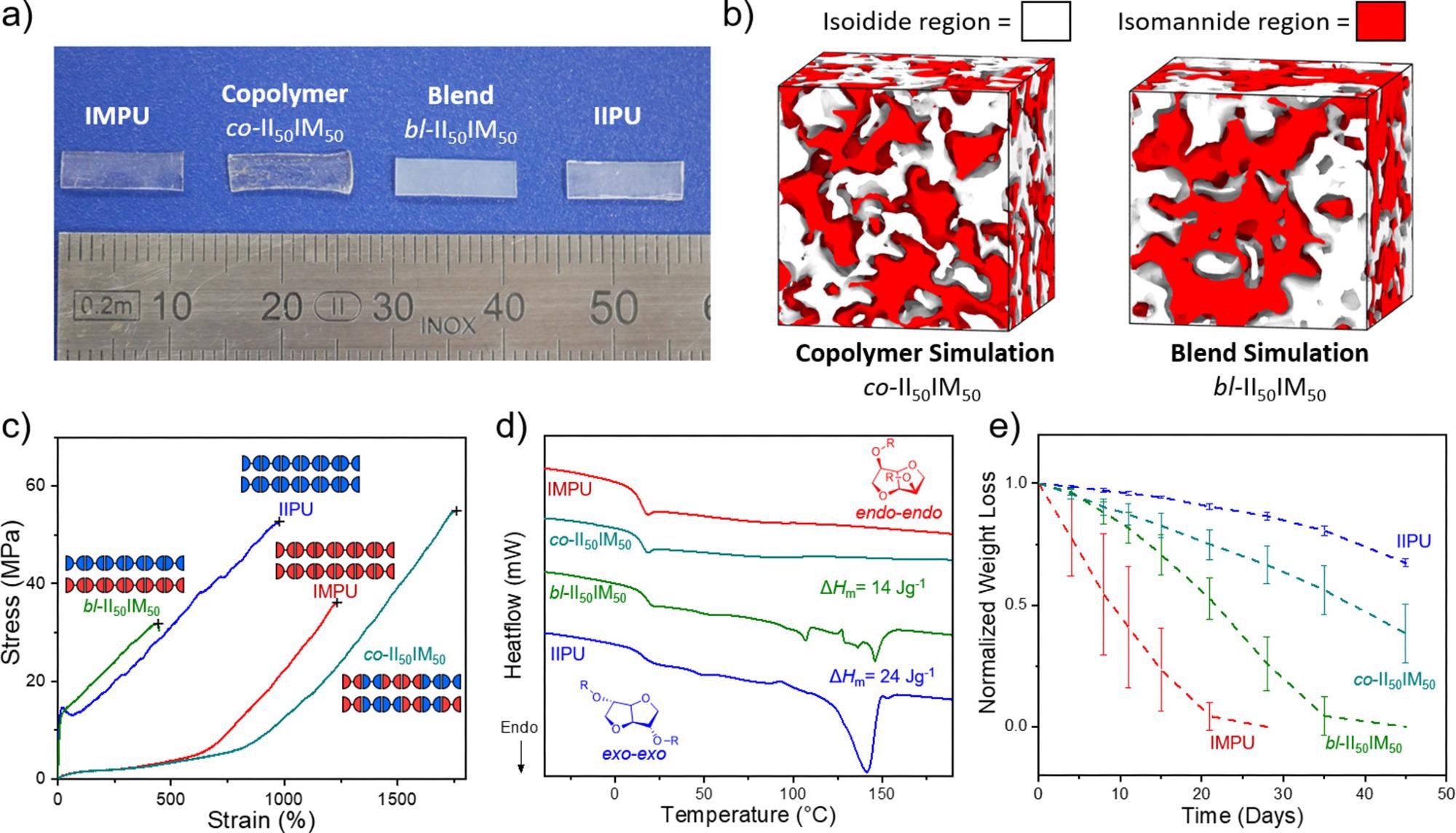
(a) Image of heat-compressed IIPU, IMPU, co-II50IM50, and bl-II50IM50 to illustrate crystallinity differences. (b) Bulk system simulations of co-II50IM50 and bl-II50IM50 illustrating the separation of isoidide (white) and isomannide (red) regions. (c) DSC thermograms of the first heating cycle of annealed IIPU, IMPU, co-II50IM50, and bl-II50IM50 at 10 K·min–1. Total enthalpy of melting (ΔHm) calculated from integration of all melting transitions. (d) Representative stress vs strain tensile curves of annealed IIPU, IMPU, and both statistical copolymers and a physical blend at 50/50 II/IM (n > 3). Mechanical testing was performed at 10 mm·min–1. (e) Normalized weight loss of IIPU, IMPU, co-II50IM50, and bl-II50IM50 discs in 1 M NaOH(aq) over 45 days at 25 °C. n = 3. (f) Table summarizing thermomechanical properties of blend and copolymer in comparison to IIPU and IMPU homopolymers: aYoung’s modulus; bultimate tensile strength (UTS); cstrain at break. Image Credit: Stubbs, C.J et al., Journal of the American Chemical Society
The Study
The study published in the Journal of the American Chemical Society has investigated producing functional polymers from isosorbide-based monomers utilizing the effects of stereochemistry.
The authors hypothesized that the bulk thermochemical properties of materials could be controlled by leveraging the different conformations of isomannide (endo-endo) and isoidide (exo-exo.) Thus, investigating this could provide an opportunity to investigate the influence of isohexide’s geometric isomerism. Additionally, the regiochemical effects which are associated with derivatives of isosorbide could be overcome.
The authors have reported the synthesis of linear polymers based on isoidide and isomannide (IIPU and IMPU) as well as their characterization. A chemical design was embraced that allows the stereochemical influence to be probed, providing pertinent information. There was a sharp contrast observed between the mechanical properties of both IIPU and IMPU, analogous to rubber and gutta-percha, with IIPU displaying plastic behavior and IMPU displaying elastomeric behavior.
From the results of the computational investigation, the authors discovered that there was a potential stereochemical influence on the material’s supramolecular hydrogen-bonding interactions. This, in turn, seems to define the mechanical properties of the polymers.
The differences in mechanical properties were further exploited by the authors by stereoisomer copolymerization and by physically mixing the stereoisomeric homopolymers. By doing so, the authors could tune the thermochemical properties and hydrolytic degradability of polymers independently.
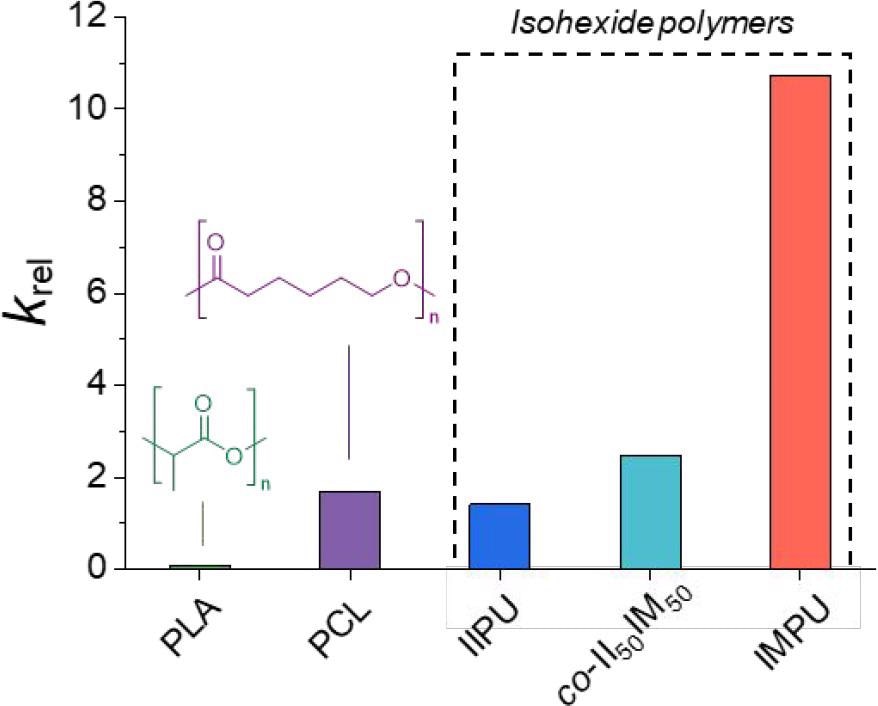
Calculated krel values for predicted seawater degradation of isohexide-based polymers and degradable commercial polymers: PLA and PCL. Image Credit: Stubbs, C.J et al., Journal of the American Chemical Society
Summarizing the Results of the Research
The team behind the new study has provided significant information on the effect of stereochemistry on polymers produced from stereoisomers of rigid-ring bicyclic ethers of sugar hexitols. The potential to leverage stereochemistry for tuning and directing supramolecular interactions is apparent in the fundamental differences in deformation behavior between polymers produced from isomannide and isoidide and their outstanding individual mechanical features.
The most intriguing feature of the system developed by the researchers is the distinct difference in properties of materials that are otherwise stoichiometrically and compositionally identical. This arises from the stereochemically distinct hydrogen bonding in these materials.
The authors have also investigated the degradation behavior of IIPU and IMPU, which is a key consideration because of the growing plastic pollution problem and the need to limit and mitigate the environmental damage caused by modern industrialized society. Quantitative and qualitative degradation profiles for the polymers were produced, which demonstrated that they degrade well in the environment. IMPU is predicted to degrade faster than IIPU, and 2 orders of magnitude faster than polylactic acid, which is a leading bioplastic.
In short, the new study published in the Journal of the American Chemical Society has demonstrated the application of stereochemistry to tune the properties of polymers derived from sugars and produce a natural-derived functional material that has a superior degradation profile in the environment. The property manipulation presented in the study is currently unparalleled amongst state-of-the-art materials and opens the possibility for polymers with on-demand tunable mechanical properties.
Further Reading
Stubbs, C.J et al. (2022) Sugar-Based Polymers with Stereochemistry-Dependent Degradability and Mechanical Properties [online] J. Am. Chem. Soc. 144(3) pp. 1243-1250 | pubs.acs.org. Available at: https://pubs.acs.org/doi/10.1021/jacs.1c10278
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.