Reviewed by Alex SmithFeb 7 2022
A group of all-RIKEN chemists has developed a polymer that exhibits the potential to catalyze its formation in an eco-friendly solvent-free process.
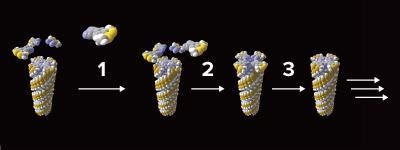 Wedge-shaped precursor molecules (top left) come together to build a rod-shaped supramolecular polymer composed of their reductively cyclotetramerized disk-shaped monomers, with each new layer of the growing polymer templating the formation of the next (1 and 2 show the preorganization of wedge-shaped precursor phthalonitrile molecules, and 3 shows reductive cyclotrimerization to give a disk-shaped phthalocyanine monomer). Image Credit: © 2022 RIKEN Center for Emergent Matter Science.
Wedge-shaped precursor molecules (top left) come together to build a rod-shaped supramolecular polymer composed of their reductively cyclotetramerized disk-shaped monomers, with each new layer of the growing polymer templating the formation of the next (1 and 2 show the preorganization of wedge-shaped precursor phthalonitrile molecules, and 3 shows reductive cyclotrimerization to give a disk-shaped phthalocyanine monomer). Image Credit: © 2022 RIKEN Center for Emergent Matter Science.
The breakthrough could result in the development of inherently recyclable polymer materials that are created using a sustainable process.
Polymers are universal, but they are harmful to the environment because of the collection of plastic waste and the unsustainable nature of traditional polymer manufacture.
Usually, polymers are created by connecting strings of building blocks together. These building blocks are called monomers and are connected by covalent bonds. However, such powerful bonds make it hard to take used, end-of-life plastic items and de-polymerize them to retrieve the monomers for reuse.
On the other hand, supramolecular polymers—composed of arrays of monomers held collectively by interactions like hydrogen bonds—are weaker, and thus more reversible, compared to covalent bonds.
But the solvents utilized for manufacturing supramolecular polymers restrict their application and sustainability.
Within two decades, solvent-free chemical manufacturing may be the only approved chemical processes, since the large volumes of solvent used in other manufacturing processes are too damaging to the environment.
Takuzo Aida, Center for Emergent Matter Science, RIKEN
Aida’s team has been analyzing wedge-shaped molecules known as phthalonitriles. At present, he and nine CEMS collaborators have found out that these substances tend to melt when subjected to heating and further form multiple thin green crystalline fibers, which look like rod-shaped supramolecular polymers.
The initiation of the polymerization process is done when four of the wedge-shaped pieces come collectively to develop a flat and circular disk. Further, the disk-shaped assembly—the monomer—could serve as a template surface for the next four wedge-shaped precursor molecules to integrate, adding a layer to the structure.
This process has been repeated several times, having each new layer of the polymer catalyzing the formation of the next, until there is a formation of long rod-like structures. The researchers called their process solvent-free autocatalytic supramolecular polymerization.
The mechanical properties of the crystalline fibers of these supramolecular polymers are analogous to those of poly(alkyl methacrylates).
Takuzo Aida, Center for Emergent Matter Science, RIKEN
Such traditional polymers are utilized for a range of applications, such as plexiglass.
The team was also able to generate more complicated versions of the supramolecular polymers by adding alternate precursor molecules into the mix at a few time points. It leads to the formation of “block copolymers” with bands of various monomers along the length of each rod.
Solvent-free autocatalytic supramolecular polymerization can be utilized to make supramolecular polymers from a range of starting materials.
It may be applicable to the synthesis of polycyclic aromatic hydrocarbons and cyclic peptides.
Takuzo Aida, Center for Emergent Matter Science, RIKEN
Journal Reference:
Chen, Z., et al. (2022) Solvent-free autocatalytic supramolecular polymerization. Nature Materials. doi.org/10.1038/s41563-021-01122-z