 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 10 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 10 2022In an article recently published in the journal ACS Central Science, researchers discussed the potential of precise carbon dioxide (CO2) reduction for the development of bilayer graphene.
Study: Precise CO2 Reduction for Bilayer Graphene. Image Credit: Rost9/Shutterstock.com
Background
C-C coupling, hydrodeoxygenation, and aromatization procedures utilize the CO2 reduction reaction (CO2RR). This reaction has gotten a lot of interest because of its importance in the recycling of CO2 into valuable chemical feedstock and investigating new CO2 transformation and utilization pathways.
Because of its unique structure and fascinating physical properties, graphene is a highly desirable product. With a configurable band-gap and suitable properties, bilayer graphene (BLG) has a lot of potential for next-generation electrical and twistronics devices.
Due to the difficulty in accurately separating the inert CO bond and the complex growth mechanism, the selective pathway from CO2 to a BLG single crystal has never been activated. By reducing molecules of CO2 into a good quality BLG single crystal, researchers can learn more about CO2 reduction while also harvesting a valuable electronic material.
When compared to standard methane (CH4), CO2 provides a safer and greener C source for graphene development. Single-layer graphene (SLG) having a single crystal structure has previously been produced from CO2 feedstock using a tandem catalytic CVD technique. However, due to insufficient oxygen content induced by uncontrolled CO2 reduction, no BLG has been reported so far. Both the quality and the pace of growth of BLG can be improved by introducing O-rich copper- and oxygen-containing compounds like ethanol.
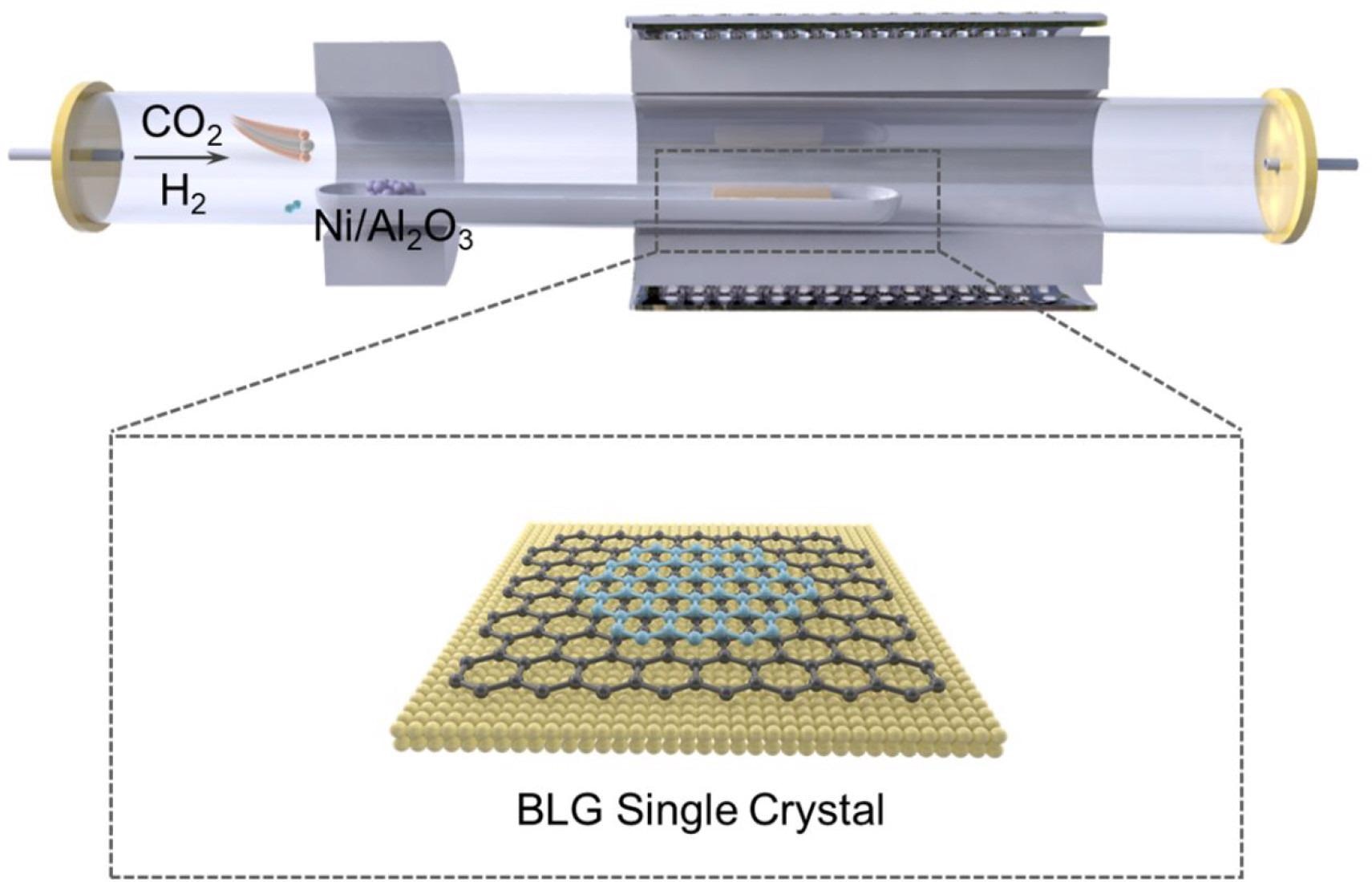
Schematic illustration of the BLG single crystal growth process. Image Credit: Gong, P et al., ACS Central Science
About the Study
In the present study, the authors presented the catalytic transformation of CO2 into a high-quality BLG single crystal with suitable mobility at ambient temperature by using a CVD approach. The CO2 molecule served as both a carbon source and an oxygen etchant in a tightly controlled development window.
By finely controlling the growth window, a catalytic approach in CVD systems was demonstrated for the precise reduction of CO2 into BLG single crystals. Huge BLG single crystals were built with exceptional rapid kinetics using CO2, wherein oxygen was used as an etching reagent, and carbon was used as a BLG growth feedstock. BLG single crystals were also used to study electrical transport parameters at room temperature.
In a tube furnace with two heating zones, the BLG single crystal was formed using the CVD process. In the first heating zone, 1.5 sccm CO2 was preliminarily activated with 200 sccm of H2 to form more reactive carbon species. This zone used a commercial Ni/Al2O3 catalyst and was kept at a temperature of 300 °C. In a subsequent tail gas chromatography (GC) setup, components of the gas mixture were quantitatively examined. The activated feed gas was then inserted into the second heating zone, where it was transformed into a BLG single crystal on a copper foil polished substrate at a temperature of 1055 °C.
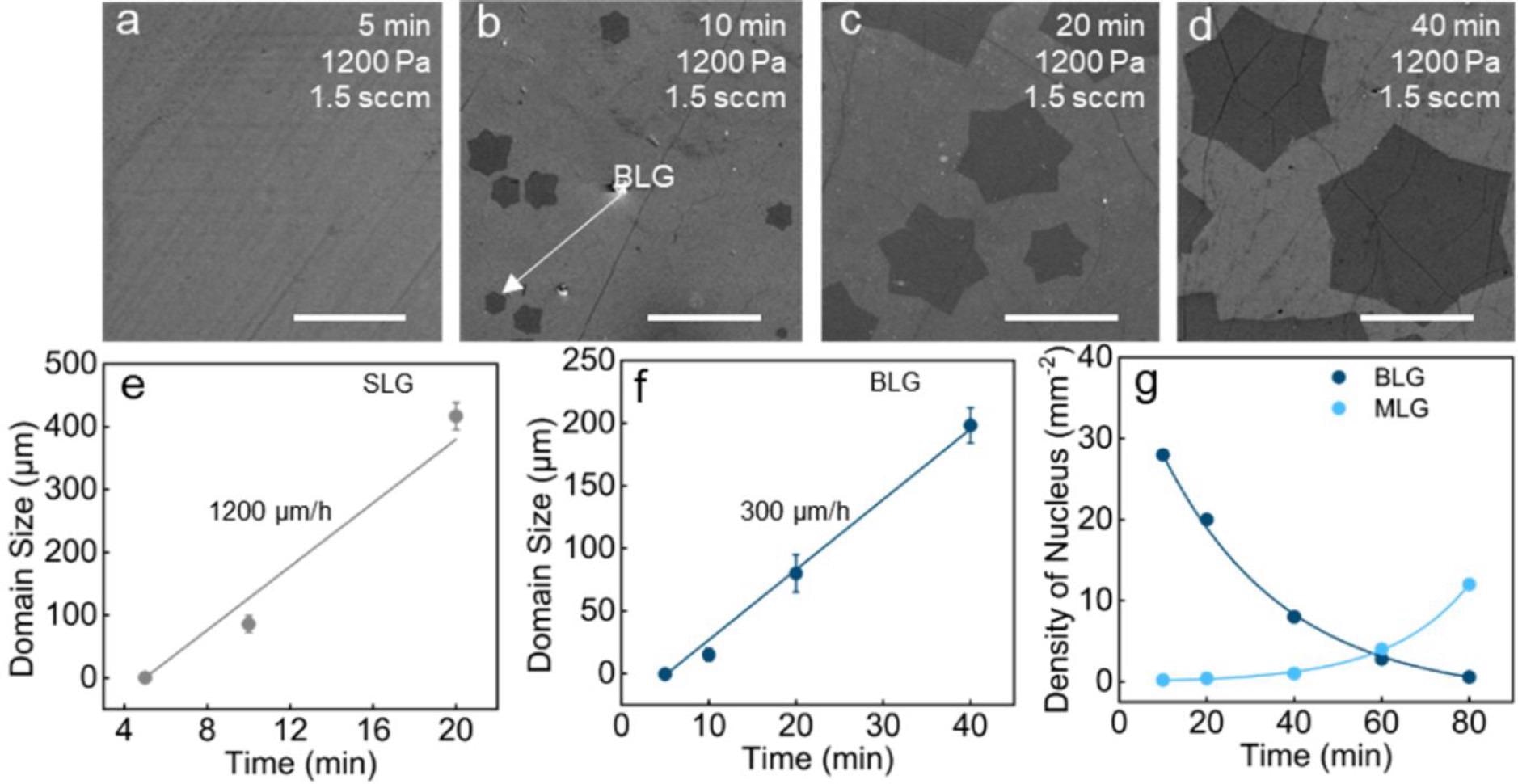
Kinetics of BLG single crystal growth. (a–d) SEM images of graphene on a 300 nm SiO2/Si substrate at 5, 10, 20, and 40 min, respectively (scale bar: 100 μm). (e) Growth rate of SLG at different reaction times. (f) Growth rate of BLG at different reaction times. (g) Density of BLG and MLG nuclei at different growth times. Image Credit: Gong, P et al., ACS Central Science
Observations
The catalytically transformed BLG crystal had a mobility of 2346 cm2 V-1 s-1 at room temperature. The BLG nucleus had a record growth rate of 300 μm h-1. The average lateral size of BLG single crystals made from CO2 was 200 μm, with an optimum value of 220 μm.
According to Raman spectra statistics, AB-stacked BLG was the most common bilayer product, accounting for 72% of all BLG single crystals, whereas the remaining 28% were primarily 30°-twisted BLG single crystals. The SLG islands united into a fully covered SLG film after 40 minutes of growth, whereas the adlayer graphene was found to grow into a bigger single crystal with a size of up to 220 μm. The pace of the first layer growth was estimated to be 1200 μm h-1 by comparing the domain size to the growth time.
After 40 minutes of growth, the coverage of BLG single crystals on the surface of SLG reached 65%. In addition, after treating the SLG film with pure CO2 at the growth conditions, an elevated D peak was found in the Raman spectrum. During the growing process, it was discovered that the growth windows for BLG and multilayer graphene (MLG) were relatively similar. The ideal growth pressure P was in the range 1100-1200 Pa, at which the BLG coverage reached its maximum but the MLG nucleation density remained low.
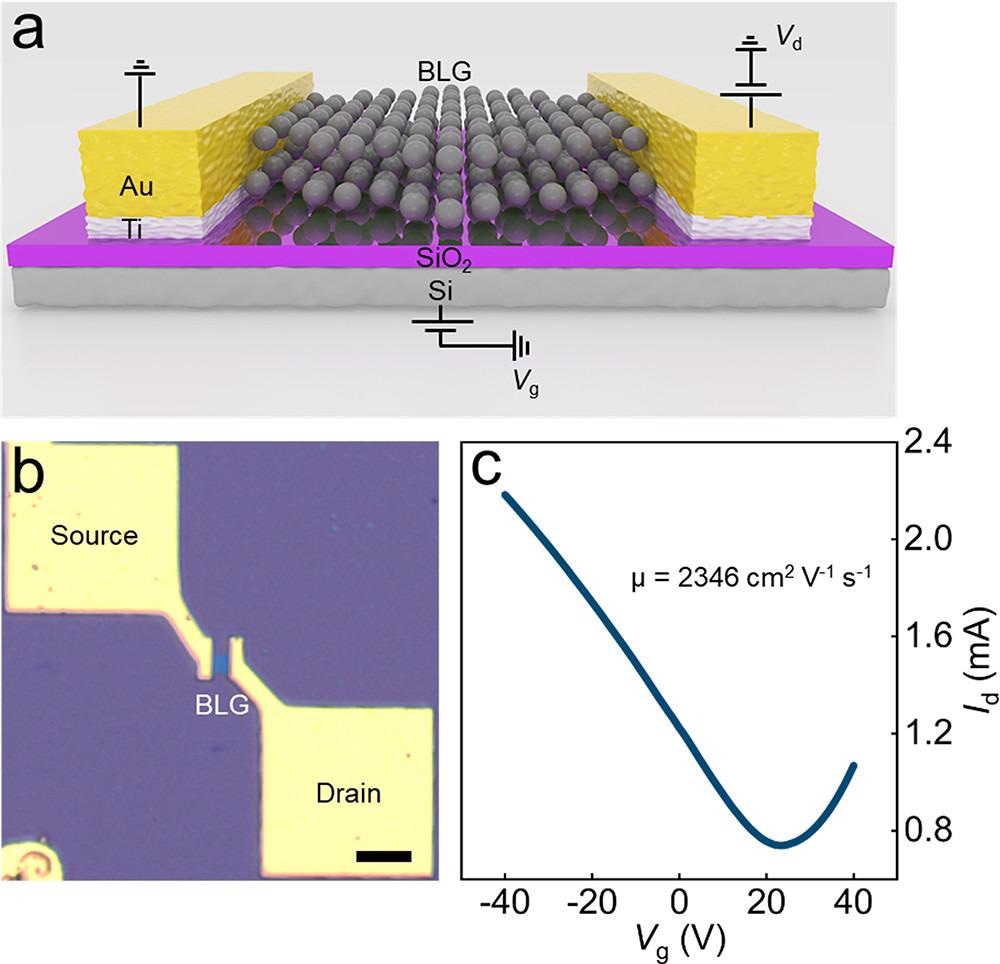
Electrical measurement of a back-gate FET device based on BLG single crystals. (a) Schematic of the back-gate BLG FET device. (b) Optical image of the BLG FET device (scale bar: 10 μm). (c) Transfer characteristic of the BLG FET device, with Vd = 1 V. Image Credit: Gong, P et al., ACS Central Science
Conclusions
In conclusion, this study demonstrated the successful development of a novel catalytic method for accurately converting CO2 into a high-quality BLG single crystal with a high growth rate, big size, and excellent electronic performance. BLG's growth rate was increased to 300 μm h-1, which was accredited to the oxygen atoms derived from CO2.
The authors emphasized that not only does an optimum concentration of etching oxygen atoms generate the proper nucleation locations for BLG, but it also prevents MLG production. They also observed that larger BLG may be grown more quickly and consistently owing to the carefully calibrated and accurate CO2 reduction, which has the potential to accelerate the development of next-generation twistronic and electrical devices.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Gong, P., Tang, C., Wang, B., et al. Precise CO2 Reduction for Bilayer Graphene. ACS Central Science (2022). https://pubs.acs.org/doi/full/10.1021/acscentsci.1c01578