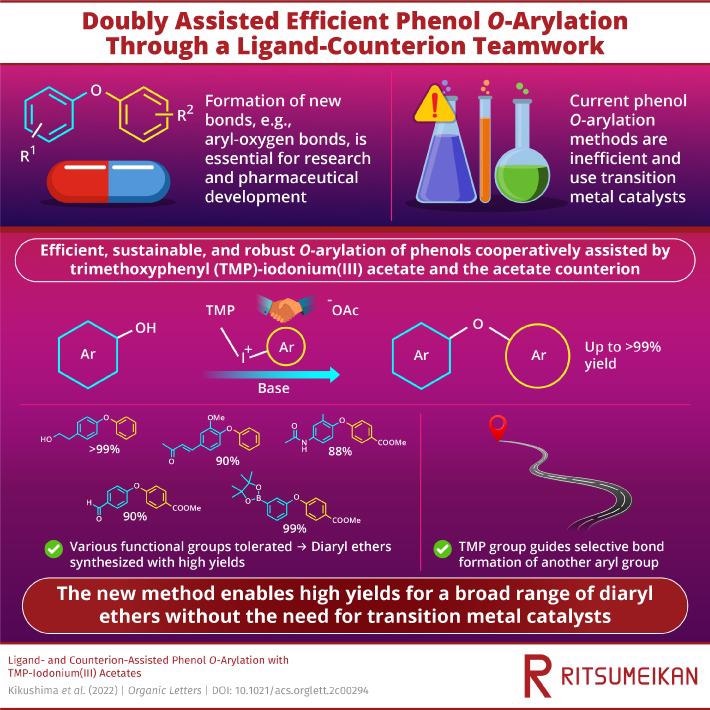
For green chemistry solutions and the pharmaceutical industry, the formation of new organic bonds in an endurable way is essential. At present, diaryl ether synthesis requires rare transition metal catalysts or a huge volume of organic solvents.
 Scientists use cooperative action of a ligand-counterion system for sustainable ether production necessary for pharmaceutical applications. Image Credit: Ritsumeikan University
Scientists use cooperative action of a ligand-counterion system for sustainable ether production necessary for pharmaceutical applications. Image Credit: Ritsumeikan University
In a new study, scientists from Ritsumeikan University have obtained high yields for an extensive range of diaryl ethers in an aqueous environment in the absence of transition metal catalysts. This is a crucial step towards a green organic chemistry future.
The constant development of pharmaceuticals relies on the potential to develop an extensive range of chemical bonds. Diaryl ethers, which have been characterized by the existence of an oxygen atom connected to two aryl groups, are known to be a class of organic compounds consisting of a wide range of applications. Diaryl ethers are considered a refrigerant and antiseptic for hindering infections.
Diaryl ethers have been a subject of research interest as their organic synthesis has proved difficult. They could be developed from aryl-alcohols, or phenols when a second aryl group has been substituted by the alcoholic hydrogen.
However, current phenol O-arylation techniques are considered to be inefficient and utilize rare transition metal catalysts (remarkably the palladium-catalyzed cross-coupling reaction won the 2010 Nobel Prize in Chemistry). They are also unselective, implying several different side products are produced, thereby decreasing the efficiency and final yield of the preferred organic compound.
Currently, a highly sustainable alternative to transition metal catalysts has been suggested by a research group from Ritsumeikan University, Japan. In this work, the transition metal has been replaced with a readily available and easily synthesized starting material, known as trimethoxyphenyl (TMP)-iodonium(III) acetate.
This iodonium salt contains two key structures, namely the TMP ligand and the acetate counterion, that work together to increase the reactivity of the O-arylation reaction and, in turn, enhance the ether bond formation, leading to significantly higher yields of diaryl ethers than has been reported in the past. It is a perfect teamwork.
Kotaro Kikushima, Study Lead Author and Assistant Professor, Ritsumeikan University
The study was published on March 18th, 2022, in the journal Organic Letters.
Depending on the structural features of phenyl (TMP)iodonium acetate, the scientists forecasted that the diaryliodonium salt would possess high reactivity. Consequently, the combination of the trimethoxyphenyl group and acetate anion collaborating to improve the reactivity of the phenol oxygen atom was identified initially in their study.
The range and diversity of compounds are considered crucial factors when developing methods for a green and sustainable chemistry future. For this method to be tested, the researchers analyzed and utilized several organic functional groups for O-arylation.
The researchers discovered that the technique was extremely strong and tolerant to a range of functional groups, resulting in a wide range of ethers synthesized with considerably higher yields compared to other reported methods, a significant consideration for industrial applications.
Furthermore, the ability to scale up this process to industrial needs has been illustrated by executing the reaction on a gram scale, thereby holding high efficiency. Besides high yields and sustainable starting materials, the technique presented one more additional benefit than the existing methods: increased selectivity.
The TMP group directed the selective arylation of the other functional group, thereby enabling more control without undesirable side products.
The present method would provide a cost-effective and robust access to a wide range of useful organic molecules under green sustainable conditions without the need for transition metal catalysts.
Toshifumi Dohi, Study Co-Author and Professor, Ritsumeikan University
Dohi stated, “Our next goal is to recycle and re-use the iodine-containing waste, which is formed as a side product during the arylation. Electrochemical or photochemical methods could then be used to sustainably restore the hypervalent iodine (III) which could then be used in another arylation.”
The addition of such green recycling plans to the presented arylation reaction would offer the perfect sustainable synthetic methodology for transition-metal-free bond formations in hazardous chemical waste, which is a seismic shift in the sustainability of organic synthesis.
With the help of splendid teamwork between ligands and counterions illustrated, the future of organic chemistry has never seemed so green.
Journal Reference:
Kikushima, K., et al. (2022) Ligand- and Counterion-Assisted Phenol O-Arylation with TMP-Iodonium(III) Acetates. Organic Letters. doi.org/10.1021/acs.orglett.2c00294.