Reviewed by Alex SmithApr 13 2022
The Nernst Equation plays a critical role in the progress of electrochemical devices like batteries. But it is only employed when there is no current flow. At present, no theory is capable of quantitatively anticipating the vital superconducting transition temperature of an electrode-electrolyte interface.
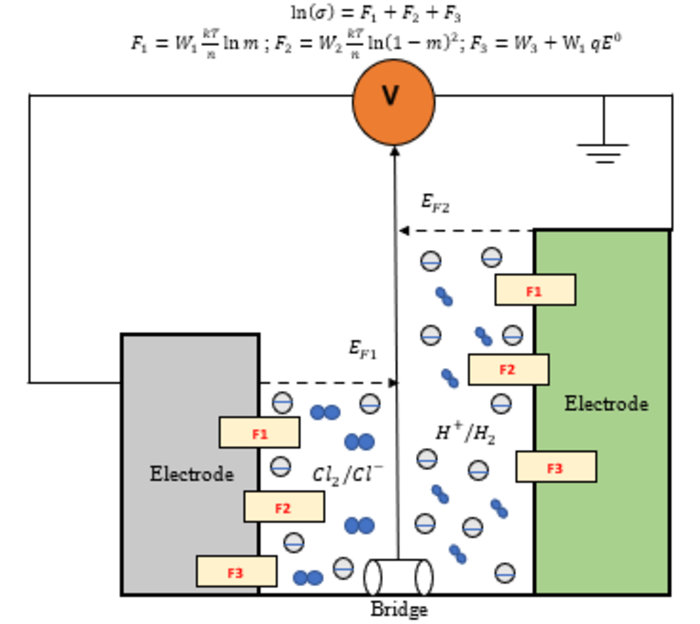 Schematic diagram of a double room electrochemical cell. Image Credit: Singapore University of Technology and Design.
Schematic diagram of a double room electrochemical cell. Image Credit: Singapore University of Technology and Design.
With the help of a conductive Nernst Equation, it is possible for this difficulty to be resolved and this would convert the technology that has been powered by superconducting and complicated gadgets and conducting batteries.
Further, it can be utilized to calculate charge transport as a function of electrolyte or electrode concentrations, redox potentials and temperature in a quantitative way. This will help in the anticipation of novel physics and the conversion of technology.
A research group from the Singapore University of Technology and Design (SUTD) has come up with first principles-based charge transport equations for electrode–electrolyte present modeling to progress the knowledge of the electrode–electrolyte transport process and fulfill the zero-current problem.
This could result in new insights and breakthroughs in electrode–electrolyte interface science, and the making of high-performance electrolytes for biomedical, energy and environmental applications, as well as prediction of the interfacial superconducting transition.
The research group created an equation of electron conductivity and electron entropy of electrodes to consider the extra overpotential and resistive loss as a result of current flow. This study gained inspiration from the success of their earlier studies on magneto-wetting, entropic electrowetting and thermodynamic entropy.
At the same time, by linking the electron electrochemical potentials of electrolyte and electrode, this equation considers the change in electrolyte chemistry at the electrode–electrolyte interfaces as a result of the current flow.
The actual power of electrochemistry was unleashed with this conductive Nernst Equation, which quantified the charge transport of electronic devices as a function of the introduced mechanical, electrical, magnetic, and chemical loadings.
Wu Ping, Study Principal Investigator and Associate Professor, Singapore University of Technology and Design
Ping added, “This conductive Nernst Equation, for example, is being used to design and control the degradation rates of human implants, as well as to investigate superconducting batteries for quick charge and discharge performance, and to explore the influence of global warming on marine organism colonies.”
The group estimates that the conductive Nernst Equation will pave the way for thrilling insights and breakthroughs in interface science. This provides exciting opportunities in the field of biochemistry, environmental chemistry and electrochemistry.
Journal Reference:
Wu, P. & Senevirathna, H. L. (2022) A charge transport prediction method for metal electrode-aqueous electrolyte interface to guide the design of metal batteries. Electrochimica Acta. doi.org/10.1016/j.electacta.2022.140051