Reviewed by Alex SmithApr 28 2022
One of the most crucial challenges in copper-dependent metalloenzymes is dioxygen activation. Copper-dependent metalloenzymes, along with lytic polysaccharide monooxygenases (LPMOs), particulate methane monooxygenases (pMMOs), and the binuclear copper enzymes PHM and DBM, can conduct a variety of complex C-H/O-H bond activations when activated by O2.
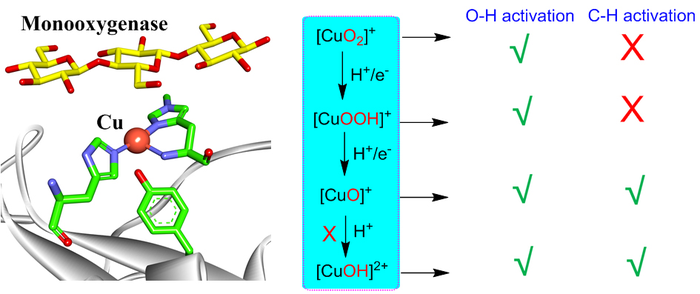 Dioxygen activation by mononuclear copper in biological and synthetic systems may generate various copper-oxygen intermediates including [CuO2]+, [CuOOH]+, [CuO]+, [CuOH]2+. All these species are able to perform O-H activation, while only [CuO]+ and [CuOH]2+ are reactive for C-H activation. However, the formation of [CuOH]2+ is highly unfavorable in monooxygenases, leaving [CuO]+ as the only active intermediate responsible for C-H activation in monooxygenases. These insights can provide consistent understanding on reactivities of various copper-oxygen active species in biological and synthetic systems. Image Credit: Chinese Journal of Catalysis
Dioxygen activation by mononuclear copper in biological and synthetic systems may generate various copper-oxygen intermediates including [CuO2]+, [CuOOH]+, [CuO]+, [CuOH]2+. All these species are able to perform O-H activation, while only [CuO]+ and [CuOH]2+ are reactive for C-H activation. However, the formation of [CuOH]2+ is highly unfavorable in monooxygenases, leaving [CuO]+ as the only active intermediate responsible for C-H activation in monooxygenases. These insights can provide consistent understanding on reactivities of various copper-oxygen active species in biological and synthetic systems. Image Credit: Chinese Journal of Catalysis
Simultaneously, numerous copper-oxygen core complexes are synthesized to imitate the metalloenzymes' active species. Dioxygen activation by a mononuclear copper active site results in a variety of copper-oxygen intermediates, notably Cu (II)-superoxo, Cu (II)-hydroperoxo, Cu (II)-oxyl, and Cu (III)-hydroxide.
Interestingly, all of these molecules are proposed as possible active intermediates for C-H/O-H activations in biological or synthetic systems. The nature of active species in both biological and synthetic systems is widely contested due to a lack of proper knowledge of the reactivities of the copper-oxygen complex.
A study team headed by Prof. Binju Wang of Xiamen University in China has recently assessed the reactivities of numerous mononuclear copper-oxygen species in both biological and synthetic systems.
The results indicate that (a) the MN15 functional is extremely accurate for mononuclear copper-oxygen complexes, where MN15 can reproduce the experimental kinetics of various C-H/O-H activations; and (b) Cu(II)-superoxo exhibits consistent reactivities in both synthetic and biological systems, being highly reactive for O-H bond activations but having low reactivities for C-H bond activations.
As a result, Cu (II)-superoxo cannot be the active species in both biological and synthetic systems for C-H activations (c) Cu (II)-hydroperoxo is inactive for C-H bond activation, but its radical character on the proximal O allows it to execute HAA from moderate O-H bonds or link with another Cu(I) to generate the dinuclear copper species.
Consequently, in both biological and synthetic systems, Cu (II)-hydroperoxo is a vital intermediate along the O2 activation routes rather than an oxidant for C-H activation. (d) Since Cu (II)-oxyl is extremely reactive for C-H bond activation, responsibility for C-H activation in mononuclear copper monoxygenases falls on it.
(e) Even though copper (III)-hydroxide has a high reactivity for C-H bond activation, the production of such species in monoxygenases is thermodynamically undesirable. These findings are likely to contribute to a more consistent comprehension of the reactivities of diverse copper-oxygen active species in biological and synthetic systems.
Journal Reference:
Wu. P., et al. (2022) Theoretical perspective on mononuclear copper-oxygen mediated C–H and O–H activations: A comparison between biological and synthetic systems. Chinese Journal of Catalysis. doi.org/10.1016/S1872-2067(21)63974-8.