Reviewed by Alex SmithApr 29 2022
The long-life span of rechargeable lithium-ion batteries is not guaranteed — even after sufficient cycles of charging and recharging. At some point, they will go kaput, so scientists are in a constant search for ways to squeeze a little more life out of their battery designs.
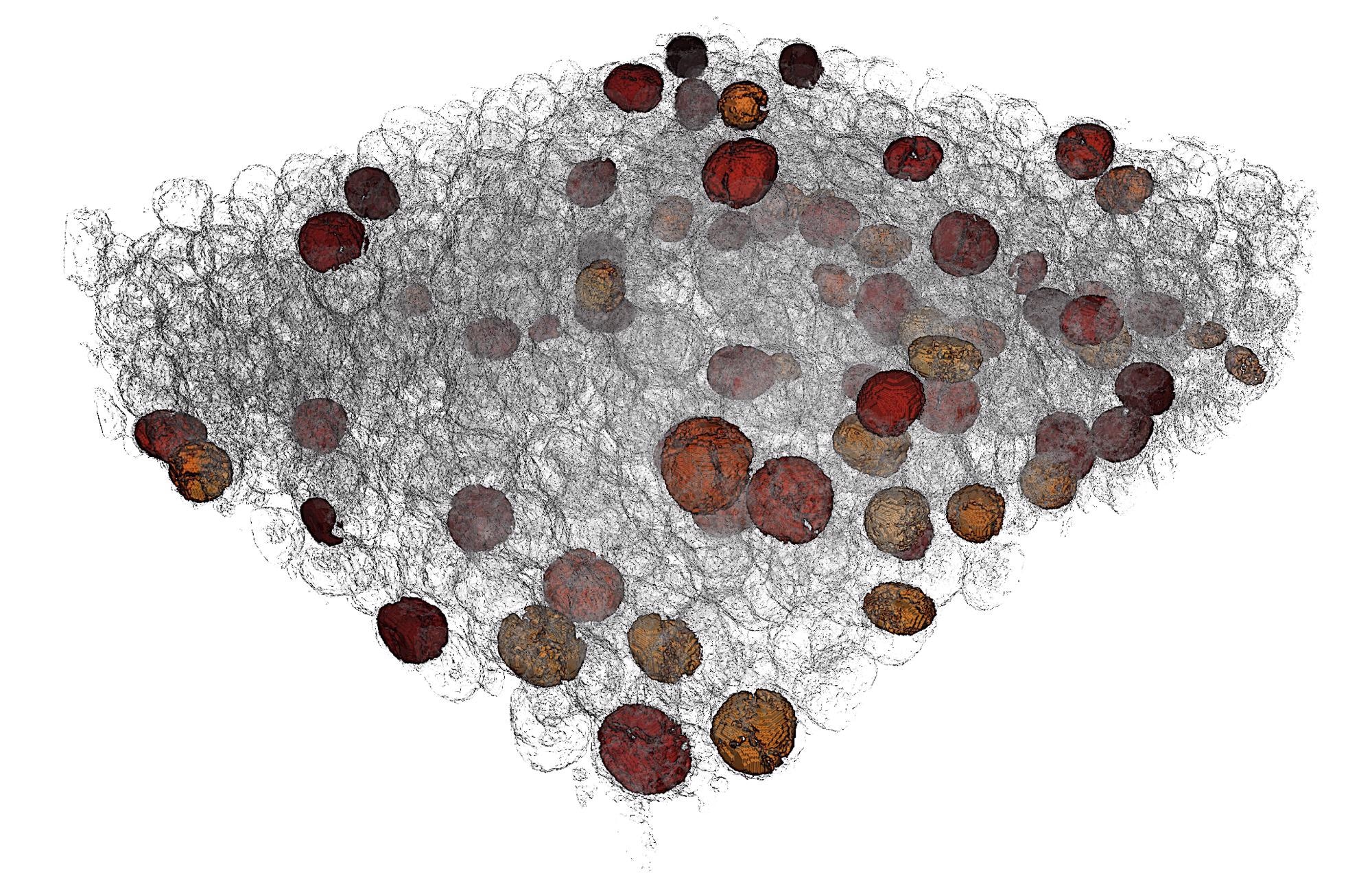 A piece of battery cathode after 10 charging cycles. A machine-learning feature detection and quantification algorithm allowed researchers to automatically single out the most severely damaged particles of interest, which are highlighted in the image. Image Credit: Yijin Liu/SLAC National Accelerator Laboratory.
A piece of battery cathode after 10 charging cycles. A machine-learning feature detection and quantification algorithm allowed researchers to automatically single out the most severely damaged particles of interest, which are highlighted in the image. Image Credit: Yijin Liu/SLAC National Accelerator Laboratory.
Currently, scientists at the Department of Energy’s SLAC National Accelerator Laboratory collaborated with Purdue University, Virginia Tech, and the European Synchrotron Radiation Facility and discovered that the factors behind battery decay tend to change with respect to time.
In the beginning, decay was occurring due to the properties of separate electrode particles. However, following various dozen charging cycles, it is the way in which those particles are combined that matters more.
The fundamental building blocks are these particles that make up the battery electrode, but when you zoom out, these particles interact with each other if you want to build a better battery, you need to look at how to put the particles together.
Yijin Liu, Study Senior Author and Researcher, Stanford Synchrotron Radiation Lightsource
Seeing the Forest for the Trees
The new study builds on an earlier study in which Liu and collaborators utilized methods of computer vision to learn how the separate particles that make up a rechargeable battery electrode tend to fall apart with respect to time. This time, the researchers aimed to investigate not only the separate particles but the ways they work collectively to extend — or degrade — the life of the battery.
The study was published on April 29th, 2022, in the Science journal
Keije Zhao, a Purdue mechanical engineering professor who works with Liu and Virginia Tech chemistry professor Feng Lin, a senior author, likened the issue to people collaborating in groups.
Battery particles are like people—we all start out going our own way. But eventually we encounter other people, and we end up in groups, going in the same direction. To understand peak efficiency, we need to study both the individual behavior of particles, and how those particles behave in groups.
Keije Zhao, Professor, Mechanical Engineering, Purdue University
For this concept to be explored, co-first authors of the study Jizhou Li, an SSRL postdoctoral fellow, and Nikhil Sharma, a Purdue graduate student, paired up with Liu, Lin and Zhao, and other collaborators studied battery cathodes along with X-Rays.
The researchers utilized X-Ray tomography to rebuild three-dimensional pictures of the cathodes following the either 10 or 50 charging cycles. Then, they split those 3D pictures into a series of 2D slices and made use of computer vision techniques to determine the particles.
A Battery’s Life
Eventually, over 2,000 individual particles were identified by the scientists, for which not only individual particle features like shape, size, and surface roughness were assessed but also more global traits, like how often particles came into direct contact with each other and how the shapes of the particles modified.
Then, the researchers observed how each of those properties added up to the particles' breakdown, and a noticeable pattern emerged. Following 10 charging cycles, the greatest factors were separate particles’ properties, inclusive of how spherical the particles were and also the ratio of particle volume to the surface area.
But following 50 cycles, pair and group attributes, like how distant two particles were, how altered their shapes were, and if more elongated, football-shaped particles were oriented in a similar way, drove particle breakdown.
It’s no longer just the particle itself. It’s particle-particle interactions.
Yijin Liu, Study Senior Author and Researcher, Stanford Synchrotron Radiation Lightsource
Liu stated that it is significant as it implies that manufacturers could engineer methods to regulate such properties. For instance, they may be able to make use of the electric or magnetic fields to arrange elongated particles with each other, which the new outcomes indicate would result in prolonged battery life.
Also, co-senior author of the study and Virginia Tech chemist Feng Lin stated that the outcomes could be employed beyond the particulars of the current study.
Lin stated, “This study really sheds light on how we can design and manufacture battery electrodes to obtain long cycle life for batteries. We are excited to implement the understanding to next-generation, low-cost, fast charging batteries.”
The study was financially supported by the DOE Laboratory Directed Research and Development program at SLAC and by the National Science Foundation. SSRL is a DOE Office of Science user facility.
Journal Reference:
Li, J., et al. (2022) Dynamics of particle network in composite battery cathodes. Science. doi.org/10.1126/science.abm8962.