Slow reaction kinetics and undesirable parasitic reactions have hampered the development of aprotic lithium-oxygen (Li–O2) batteries with ultra-high theoretical specific energies.
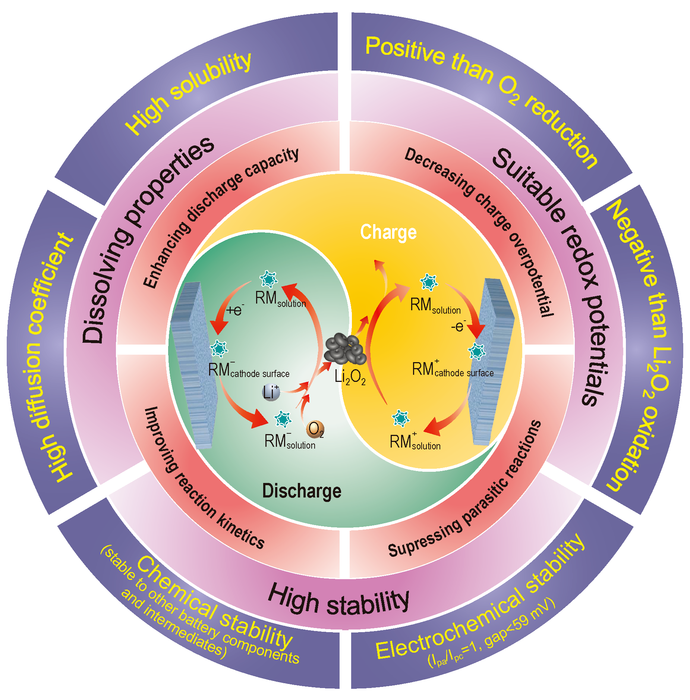 Catalytic mechanism during oxygen reduction reaction and oxygen evolution reaction, unique advantages and corresponding screening criteria of redox mediators for lithium-oxygen batteries. Image Credit: Science China Press.
Catalytic mechanism during oxygen reduction reaction and oxygen evolution reaction, unique advantages and corresponding screening criteria of redox mediators for lithium-oxygen batteries. Image Credit: Science China Press.
Molecular catalysts, such as redox mediators (RMs), have been investigated as a potential technique for catalyzing oxygen electrochemistry in Li–O2 batteries.
Dr. Yaying Dou (Zhengzhou University), Dr. Zhaojun Xie (Nankai University), Prof. Zhen Zhou (Zhengzhou University), Prof. Yingjin Wei (Jilin University), and Prof. Zhangquan Peng (Dalian Institute of Chemistry, Chinese Academy of Sciences) recently summarized the development and application of the recently updated RMs in Li–O2 batteries.
The operating principles, unique benefits and selection criteria of RMs in Li–O2 batteries are highlighted by Dr. Dou and others. They classified RMs into organic, organometallic, and inorganic substances depending on their chemical composition while discussing recent developments in RMs.
Meanwhile, they categorize RM catalytic functions into three categories based on the catalyzed electrode reactions: ORR RMs, OER RMs, and bifunctional RMs. On this basis, they outline the catalytic processes and corresponding optimization strategies for RMs.
To be objective, although RMs provide new prospects for Li–O2 batteries, the scientific issues and technical challenges raised cannot be ignored.
Dr. Yaying Dou, Zhengzhou University
The compatibility of RMs with other battery components (electrode materials, solvents, salts, and so on) is unknown. Furthermore, RMs’ redox shuttle causes Li anode degradation and RMs’ catalytic activity to be lost.
Some organic RMs may decompose in the same way as electrolytes or carbon. Above all, there is no agreement on the parameters that influence the kinetics of the interaction between RMs and reactants.
The team made recommendations for future research on RMs, including acknowledging the oxidation kinetics of Li2O2 with RMs, governing the molecular structure of RMs, enhancing the components of RMs-assisted Li–O2 batteries, evaluating the catalytic efficiency of RMs, and discovering the guidelines for finding new RMs, all of which are based on a dual background of academic exploration and practical application.
Furthermore, they show that practical Li–O2 battery research is primarily focused on three aspects: basic principles behind Li–O2 electrochemistry, further optimization of battery components, and attaining Li–O2 battery functioning in ambient circumstances.
Dr. Dou added, “More advanced experimental, computational, and applied investigations are needed to advance practical development of RM-assisted Li–O2 batteries.”
Journal Reference:
Dou, Y., et al. (2022) Redox mediators for high-performance lithium–oxygen batteries. Nat Sci Rev doi:10.1093/nsr/nwac040.