Reviewed by Alex SmithMay 23 2022
Rechargeable battery technology powers digital lifestyles while also allowing renewable energy to be integrated into the power grid. However, battery function in cold temperatures remains a challenge, prompting research into improving battery low-temperature performance.
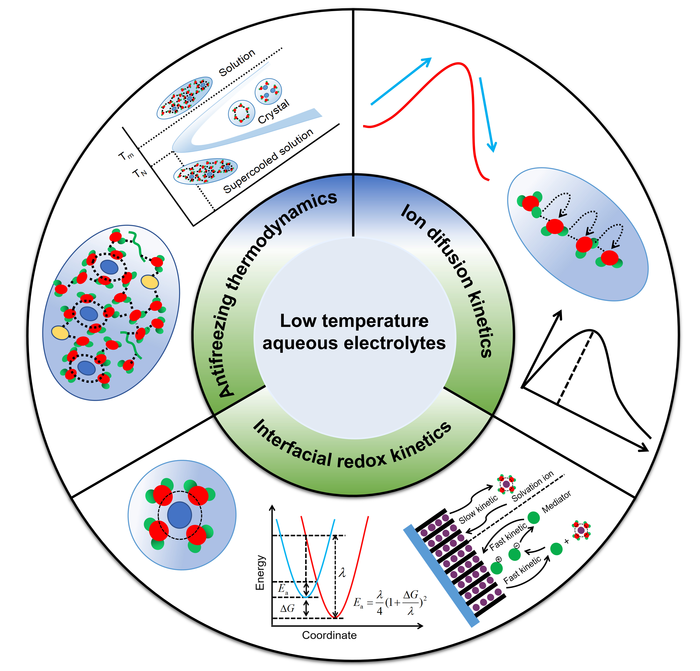
Diagram showing design strategies for aqueous electrolytes, including antifreezing thermodynamics, ion diffusion kinetics, and interfacial redox kinetics. Image Credit: Nano Research Energy.
At low temperatures, aqueous batteries (in a liquid solution) outperform non-aqueous batteries in terms of rate capability (the amount of energy discharged per unit of time).
Engineers from the China University of Hong Kong have proposed optimal design elements for aqueous electrolytes to be used in low-temperature aqueous batteries. The research was published on April 17th, 2022, in the Nano Research Energy journal.
The study examines the physicochemical properties of aqueous electrolytes (which influence their battery performance) using several metrics, including ion diffusion rates, phase diagrams, and kinetics of the redox reactions.
The electrolytes freeze, the ions diffuse gradually, and the redox kinetics (electron transfer mechanisms) are thus sluggish in low-temperature aqueous batteries. The physicochemical properties of the low-temperature aqueous electrolytes used in batteries are strongly connected to these parameters.
Insight into how the electrolytes react to cold (–50 °C to –95 °C) is required to enhance battery performance in cold conditions.
To obtain high performance low temperature aqueous batteries (LT-ABs), it is important to investigate the temperature dependent physicochemical properties of aqueous electrolytes to guide the design of low temperature aqueous electrolytes (LT-AEs).
Yi-Chun Lu, Study Author and Associate Professor, China University of Hong Kong
Evaluating Aqueous Electrolytes
The authors examined aqueous Li+/Na+/K+/H+/Zn2+ supercapacitors, batteries, and flow batteries, as well as other LT-AEs used in energy storage technologies. The research compiled data from a number of other reports on the performance of various LT-AEs, such as an antifreezing hydrogel electrolyte for an aqueous Zn/MnO2 battery; and an ethylene glycol (EG)-H2O based hybrid electrolyte for a Zn metal battery.
To better understand the antifreezing mechanisms of these reported LT-AEs, they looked at equilibrium and non-equilibrium phase diagrams. The phase diagrams demonstrated how the electrolyte phase changes as temperature changes. The researchers looked at LT-AE conductivity in relation to temperature, electrolyte concentrations, and charge carriers.
Ideal antifreezing aqueous electrolytes should not only exhibit low freezing temperature Tm but also possess strong supercooling ability.
Yi-Chun Lu, Study Author and Associate Professor, China University of Hong Kong
This indicates that the liquid electrolyte medium remains liquid even below freezing temperature, thus facilitating ion transport at ultra-low temperature.
The researchers discovered that the LT-AEs that allow batteries to function at ultralow temperatures have low freezing points and robust supercooling abilities.
Lu adds, “The strong supercooling ability can be realized by improving the minimum crystallization time τ and increasing the ratio value of glass transition temperature and freezing temperature (Tg/Tm) of electrolytes.”
Reducing the amount of energy needed for ion transfer, tweaking the concentration of electrolytes, and selecting charge carriers that enhance fast redox reaction rates could optimize the charge conductivity of the reported LT-AEs for use in batteries.
Lowering the diffusion activation energy, optimizing electrolyte concentration, choosing charge carriers with low hydrated radius, and designing concerted diffusion mechanism[s] would be effective strategies to improve the ionic conductivity of LT-AEs.
Yi-Chun Lu, Study Author and Associate Professor, China University of Hong Kong
The researchers plan to investigate the physicochemical properties of electrolytes that make a contribution to enhanced aqueous battery performance at low temperatures in the future.
Lu concludes, “We would like to develop high-performance low-temperature aqueous batteries (LT-ABs) by designing aqueous electrolytes possessing low freezing temperature, strong supercooling ability, high ionic conductivity, and fast interfacial redox kinetic.”
The study was financially supported by the Research Grant Council of the Hong Kong Special Administrative Region, China.
Journal Reference:
Jiang, L., et al. (2022) Design strategies for low temperature aqueous electrolytes. Nano Research Energy. doi.org/10.26599/NRE.2022.9120003.