A paper recently published in the journal Materials demonstrated the commercial viability of upcycling pharmaceutical glass into ceramics.

Study: Upcycling of Pharmaceutical Glass into Highly Porous Ceramics: From Foams to Membranes. Image Credit: sspopov/Shutterstock.com
Background
Pharmaceutical industries have traditionally relied on borosilicate glass vials owing to their high chemical stability and indefinite ‘theoretical’ recyclability. However, the recyclability cannot be realized practically for these glasses as they are prepared from high purity feedstock and then shaped into different preforms, such as rods and tubes, in highly specialized facilities.
Closed-loop recycling that involves remelting the disposed glass waste to obtain new glass articles is extremely challenging. However, open-loop recycling involving the reuse of the disposed glass as a raw material for the production of new marketable products can lead to several advantages, such as the reduction in the utilization of natural raw materials and environmental dispersion of glass waste.
An extremely sustainable waste management model can be configured if the revenues from the marketable products can compensate for the costs of transforming the waste/discarded glass into these products. However, the economic value of the synthesized products must be high compared to their manufacturing costs to achieve sustainable upcycling through open-loop recycling.
Glass foams can be considered a model product of open-loop glass recycling due to their significantly higher stability than polymer foams. Finely powdered glass material is subjected to viscous flow sintering and gas evolution from specific foaming agents to synthesize the glass foams.
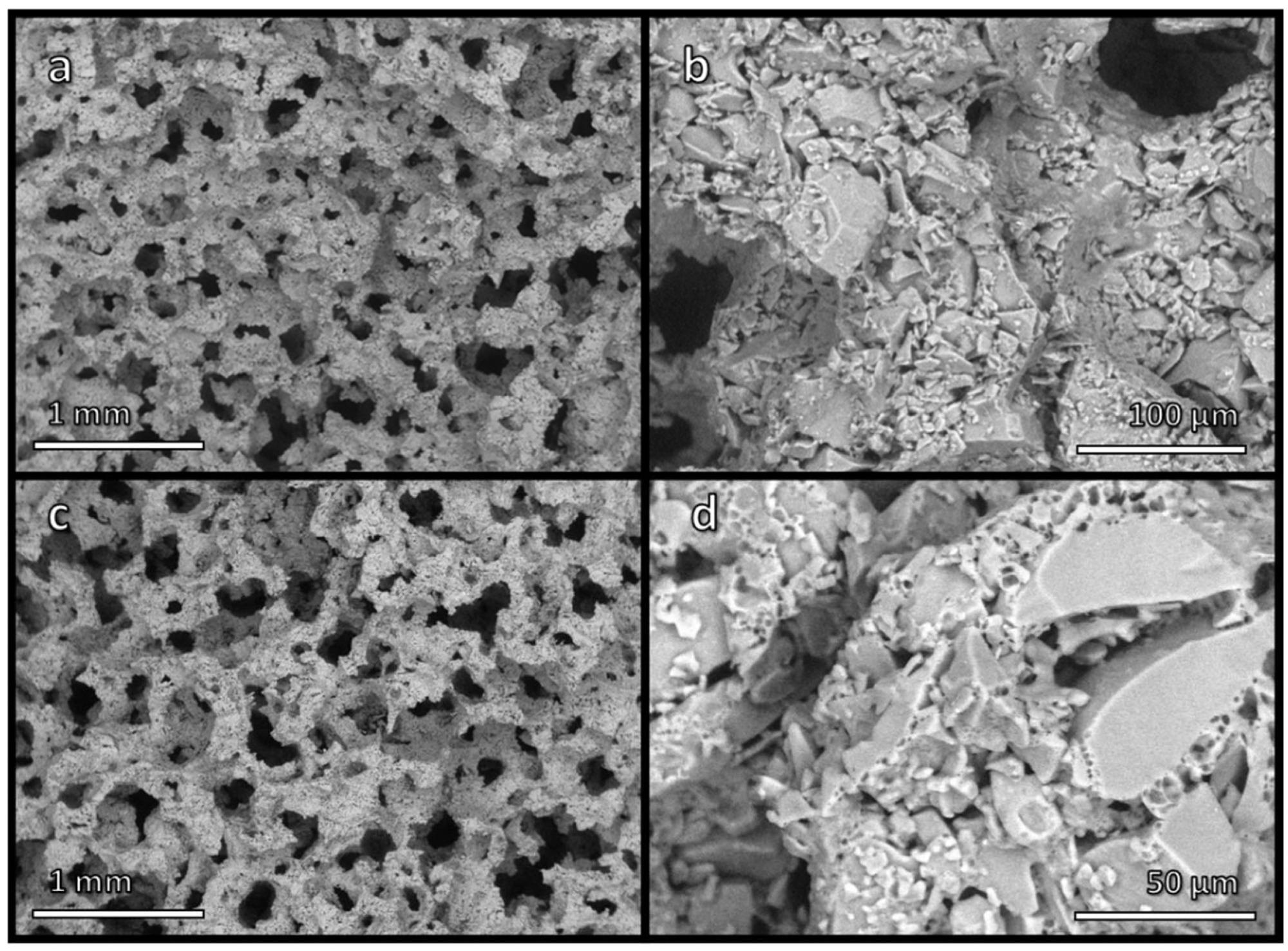
Low and high magnification micrographs of (a,b) foamed suspension of glass particles in alkaline solution, after drying; (c,d) glass foam, after firing at 650 °C. Image Credit: Mehta, A et al., Materials
These foams can be used as thermally insulating materials, which ensures the overall sustainability of this open-recycling model since the manufacturing costs are compensated by the energy savings achieved during their long service duration.
However, the need for a high processing temperature from 850-900 oC compared to the minimum viscous flow sintering temperature of 700 oC for soda-lime glass and the high cost of foaming agents are the major challenges in this model.
Recently, an alternative method was used to prepare green glass foams where the viscous flow sintering was applied to coalesce the cellular structures synthesized at room temperature using the gel casting method. Green foams were obtained by a vigorous mechanical stirring of alkali-activated glass powder aqueous suspensions with added surfactants, leading to progressive gelation.
The firing of the dried glass powder suspensions can be performed at low temperatures, such as at 700 oC for soda-lime glass. Although highly porous cellular bodies were well-defined before the firing, certain reshaping of pores can occur due to the impact of the decomposition of compounds used for gelation. These compounds remained as binding phases after drying.
Calcium silicate hydrated (C-S-H) compounds are formed as binding phases at the surface of the glass due to the alkali activation of bioactive glasses and soda-lime glass. Although calcium oxide-free pharmaceutical glass does not facilitate the formation of such C-S-H compounds, the gelation of alkali-activated pharmaceutical glass suspensions is both possible and applicable for the synthesis of glass foams.
Pharmaceutical glass suspensions harden during gelation due to the formation of hydrated alkali alumino-silicate zeolite-like gels owing to the boro-alumino-silicate chemical formulation of the glass. These gels are similar to alkali-activated materials (AAM) such as geopolymers.
The Study
In this study, researchers examined the opportunities and challenges for the upcycling of pharmaceutical glass through the gel casting technique. Unlike the previous experiments, the surfactant amount and temperature were reduced after alkali activation and then eliminated.
The elimination of alkali carbonates after boiling water was studied thoroughly. The unfired and unfoamed hardened samples were utilized as adsorbents to evaluate their effectiveness in eliminating methylene blue dye. Lastly, researchers incorporated titanium dioxide in the glass powder to determine the effectiveness of the composite as a photocatalyst.
Pharmaceutical boro-alumino-silicate glass powder obtained by crushing disposed pharmaceutical vials was used as the starting material for the study. Initially, the glass vials were ball milled and sieved to obtain glass particles with less than 75 µm diameter.
The fine particles were then added to an aqueous solution of sodium hydroxide and potassium hydroxide and chemically treated for four h in the solution under mechanical stirring at low speed. Subsequently, the alkali-activated partially dissolved glass powder suspensions were cast in closed cylindrical polystyrene molds and then cured for two h at 75 oC.
After curing, Triton X-100 surfactant was added to the suspensions, and the resultant mixture was subjected to vigorous mechanical stirring at 2000 rpm. Subsequently, the foamed suspensions were left for 24 h at 40 oC to obtain green foams, which were then heat-treated at 550–650 oC for one h at a 10 oC/min heating rate. Selected mixtures without the surfactant Triton X-100 remained unfoamed, while certain mixtures with Triton X-100 were low fired at 550 oC and unfired.
X-ray diffraction (XRD) method, Fourier transform infrared spectroscopy, helium pycnometer, universal testing machine, scanning electron microscopy, and an ultraviolet-visible (UV-Vis) spectrophotometer were used for the characterization of different synthesized samples.
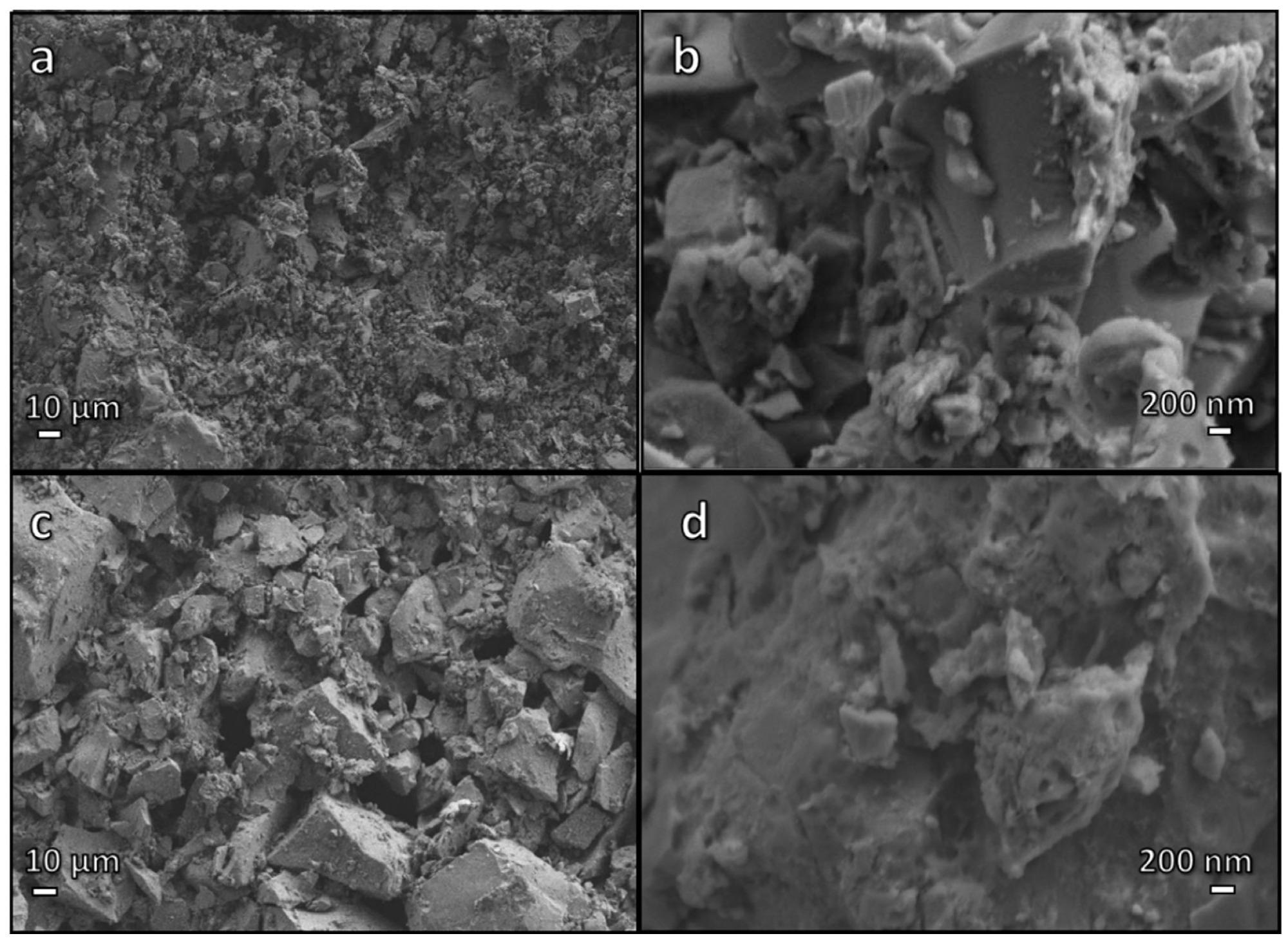
Low and high magnification micrographs of samples after cold consolidation: (a,b) simply hardened suspension; (c,d) after boiling test. Image Credit: Mehta, A et al., Materials
Observations
Different porous ceramics were synthesized from the upcycling of pharmaceutical glass. Green glass foams were manufactured successfully using the gel casting method. Highly uniform cellular structures were obtained by an intensive mechanical stirring of partially gelified pharmaceutical suspensions with added surfactant and then drying and firing the mixture at 550–650 oC.
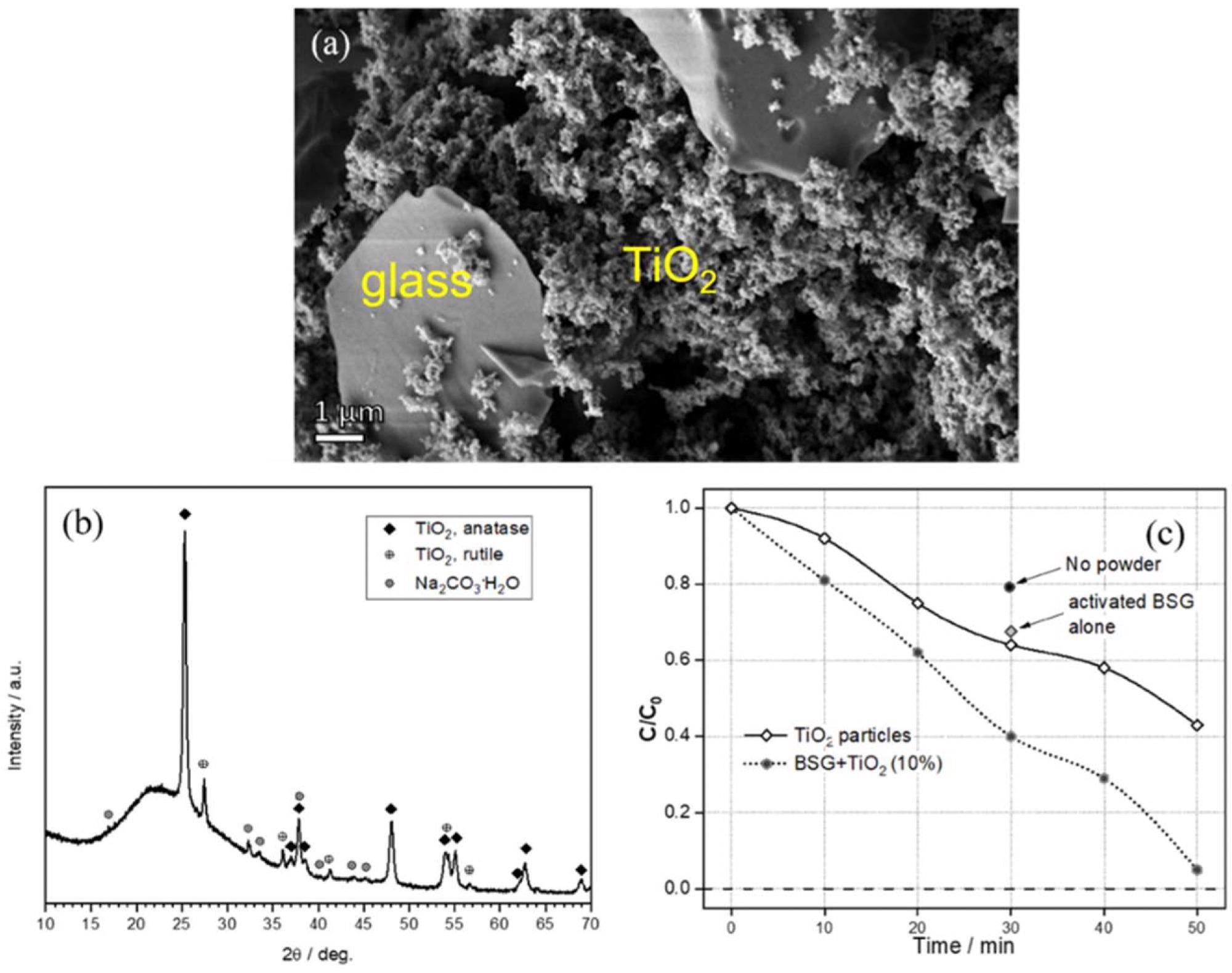
(a) Detail of glass/TiO2 composite granule; (b) diffraction analysis of hardened glass/TiO2 suspension; (c) evolution of relative concentration with increasing UV exposition time operating with TiO2 photocatalyst. Image Credit: Mehta, A et al., Materials
The alkali-activated pharmaceutical boro-alumino-silicate glass displayed considerable potential for an extensive range of applications. The interactions between the alkaline solution and glass yielded phases that played a multiform role.
The interactions yielded a liquid phase upon heating that enabled the sintering of glass at temperatures much lower than the glass transition temperature (Tg) needed for viscous flow sintering. Moreover, the interactions also facilitated the consolidation of glass foams without firing.
Cellular structure stabilization at temperatures below the Tg of the pharmaceutical glass was enabled by the thermal decomposition of the gel phase in place of viscous flow sintering of glass. This phenomenon facilitated the synthesis of glass membranes with 78 vol% open porosity by the direct firing of hardened glass suspensions without mechanical stirring and surfactant addition.
Additionally, the materials synthesized by cold consolidation of pharmaceutical glass powders at room temperature could be used for manufacturing new-generation glass membranes. These materials could also be used as sorbents for photodegradation/removal of dyes and as photocatalysts by including titanium dioxide. The alkali separation was observed in the form of hydrated carbonate phases, which was favorable for the stability of gels that act as binders for the glass particles.
To summarize, the findings of this study demonstrated an economically viable way to upcycle discarded pharmaceutical glass into several products, including highly porous ceramics, for high value-added applications.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Mehta, A., Karbouche, K., Kraxner, J. et al. Upcycling of Pharmaceutical Glass into Highly Porous Ceramics: From Foams to Membranes. Materials 2022. https://www.mdpi.com/1996-1944/15/11/3784