Most manufacturing businesses rely on the synthesis of organic chemicals and polymers. The new “electrifying synthesis” technologies, which combine traditional synthetic chemistry and electrochemistry, bring us a step closer to a more sustainable future.
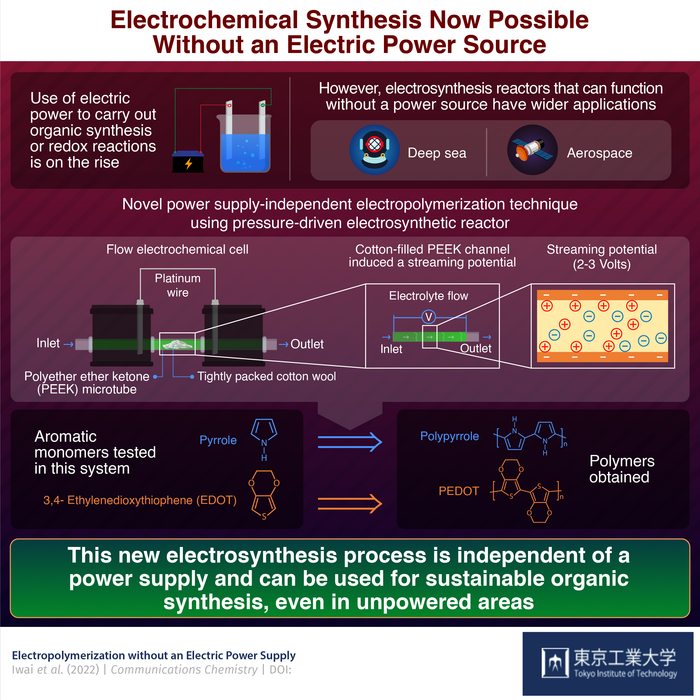
Image Credit: Tokyo Institute of Technology.
These reactions do not necessitate the use of potentially dangerous chemical reagents. They perform redox reactions with electrons from an electric power source to achieve chemical synthesis.
These reactions can be made more or less specific by fine-tuning the electric possibilities, in addition to being ecologically benign. However, their reliance on a power supply limits its use in unpowered environments such as aerospace and deep water.
Professor Shinsuke Inagi of the Tokyo Institute of Technology (Tokyo Tech) in Japan led a group of academics who revealed the answer to this self-contradictory challenge. The team recently published clear evidence for electrochemical polymerization of organic aromatic monomers without the use of an external power source in the journal Communications Chemistry.
We have seen a huge leap in the development of electrochemical reactors for carrying out organic synthesis, but most of them require a power source. We wanted to build a power-independent system to make the process more accessible. And we found the answer to our quest in streaming potential-driven electrochemistry.
Shinsuke Inagi, Professor, Tokyo Institute of Technology
What exactly does Professor Inagi mean by “streaming potential?”
Due to the movement of an electrolyte through a microchannel, a pressure difference is formed. This causes a charge imbalance, which leads to the emergence of a streaming perspective. For their studies, the team used a unique two-chambered polyether ether ketone (or PEEK) cell connected by platinum wires and a PEEK microtube.
To induce a pressure drop, this PEEK microtube was firmly filled with cotton wool. They created a streaming potential by passing an electrolyte through the microtube, which might provide enough energy to drive the appropriate chemical reactions.
The electrodes in the two-chambered cell experienced both upstream and downstream streaming possibilities when the cell was run, allowing the cell to act like a split bipolar electrode (BPE). This BPE system, in combination with the generated streaming potential of 2-3 volts, was responsible for providing favorable circumstances for organic monomer redox reactions.
The researchers picked two aromatic organic compounds to evaluate the polymerization properties of this configuration: Pyrrole (Py) and 3, 4-Ethylenedioxythiophene (EDOT). Without the need for any external power supply, both of these monomers were effectively electropolymerized into polypyrrole (PPy) and poly-EDOT (PEDOT), respectively.
This innovative pressure-driven, energy-efficient, and power-independent reactor opens up new possibilities for electrifying chemical reactions. The results of this study could be useful in the development of novel electrochemical reactors for the production of useful organic compounds and polymers.
The entire world is trying to make essential industrial processes greener and cleaner. Since organic synthesis is at the heart of many chemical industries, we tried to develop an electrosynthesis process that requires minimum resources and contributes towards the sustainable development goals.
Shinsuke Inagi, Professor, Tokyo Institute of Technology
Journal Reference:
Iwai, S., et al. (2022) Electropolymerization without an electric power supply. Communications Chemistry. doi.org/10.1038/s42004-022-00682-8.