Reviewed by Alex SmithJun 6 2022
Tetraphenylammonium, comprising all four hydrogens of ammonium (NH4+) substituted with benzene rings, has neither been found in nature nor chemically manufactured, raising the question of whether it could occur.
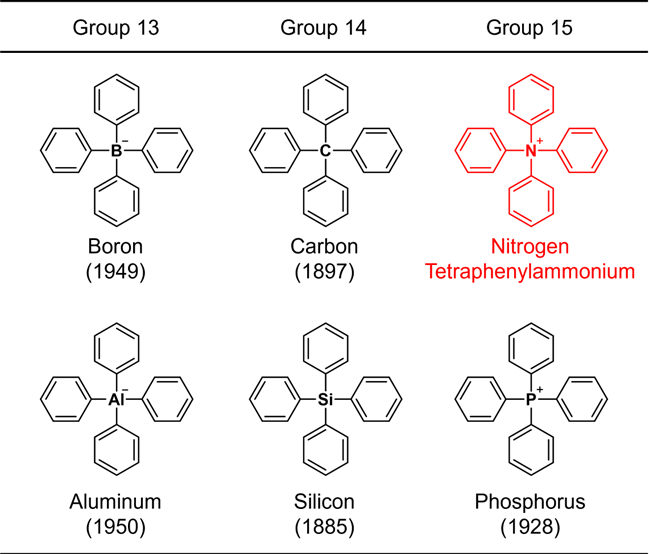 Structures of tetraphenyl-substituted elements belonging to the groups 13–15. The number in parentheses indicates the year of synthesis. Image Credit: Kanazawa University.
Structures of tetraphenyl-substituted elements belonging to the groups 13–15. The number in parentheses indicates the year of synthesis. Image Credit: Kanazawa University.
In this study, the researchers have been successful in manufacturing tetraphenylammonium for the first time, illustrating its stable existence. Radical coupling was the synthetic approach used in this research, which could be applicable to the production of many related ammoniums with high structural uniqueness.
Background
As the benzene ring is a fundamental constituent of organic compounds, a structure containing only a standard element along with the benzene ring is said to be one of the most basic chemical skeletons.
Owing to their significance, the chemical synthesis of these types of molecules has been explored since the initial days of organic chemistry. For instance, the structure wherein four benzene rings are bonded to a typical element (carbon, boron, silicon, aluminum, or phosphorus) of groups 13 to 15 in the periodic table was produced over 7 decades ago, and the oldest synthetic report goes back 137 years.
These skeletons are jointly called "tetraphenyl" structures by affixing "tetra" denoting "4" to "phenyl"), which means that the structure comprises four benzene rings. When the core element is nitrogen, ammonium, NH4+, is viewed as the parent ion.
Such a compound is referred to as tetraphenylammonium. This compound, actually an ion, has a very basic chemical structure that even a novice in organic chemistry can certainly imagine.
However, it has been determined to be very challenging to synthetically develop this structure, and no synthetic reports with precise structure identification have been reported. Moreover, as it has not been found in nature, it was not clear until now if tetraphenylammonium can occur at all.
Studies have been reported that assume its existence and comment only on its use without illustrating its production or acquisition technique. Compound databases comprise only the chemical structure. Therefore, this ion is occasionally referred to as if it were already known. However, no one has ever actually observed it, rendering tetraphenylammonium a “phantom ion.”
Results
In the present study, researchers from the Faculty of Pharmaceutical Sciences, Kanazawa University have facilitated the synthesis of tetraphenylammonium by formulating a unique synthetic approach. The critical point in the making of tetraphenylammonium is the incorporation of the fourth phenyl group into the nitrogen atom that already has three phenyl groups linked.
It was believed to be tough to accomplish this creation with conventional methods. In the current research, therefore, the researchers applied a method known as radical coupling and used an approach of reacting the radical cation 1 prepared from a triphenylamine derivative with the phenyl radical 2.
Consequently, although the production was as low as 0.1%, the researchers were successful in realizing the anticipated chemical conversion. In these types of radical couplings, extremely reactive radicals form bonds with each other, which has the benefit of supporting bond formation that could not be accomplished using other approaches.
Conversely, it has a drawback in the sense that it is tough to regulate as the reactivity is very high, resulting in many side reactions. Thus, in this production, to overpower as much as possible the side reaction of bond formation on the carbon of radical cation 1, the researchers also planned the introduction of protecting groups that trigger steric hindrance.
Finally, five steps of chemical conversion of an identified triphenylamine derivative, the starting material for the production, were performed via the addition of the protecting groups, radical coupling, and ensuing elimination of the protecting groups, resulting in tetraphenylammonium.
Based on the data acquired from several instrumental analyses, the structure of tetraphenylammonium was verified. X-Ray crystallography exposed that the bond length between the nitrogen atom and the phenyl group carbon atom held in this ion is just 1.529 Å.
Since this bond length is smaller than that of a tetraphenyl structure comprising another element (silicon, boron, aluminum, carbon, or phosphorus), it is obvious that tetraphenylammonium’s nitrogen atom is in a more spatially obstructed environment than other elements. This three-dimensional (3D) obstruction is said to be one of the features that make it tough to build this skeleton.
Furthermore, the study’s results also exposed that tetraphenylammonium possesses high stability to endure strong acidic and basic surroundings.
Future Prospects
This study has shown that tetraphenylammonium does actually exist and can be chemically made. If the mass-production of this ion and its derivatives is accomplished in the future, it could possibly be applied in several research areas as an organic cation possessing high chemical stability.
Additionally, the radical coupling approach used in this research could be applied in the synthesis of other associated ammoniums that could not be created thus far.
Journal Reference:
Fujita, H., et al. (2022) Synthesis and characterization of tetraphenylammonium salts. Nature Communications. doi.org/10.1038/s41467-022-30282-y.