Reviewed by Alex SmithJun 21 2022
Prof. Huigang Zhang of the Chinese Academy of Sciences’ Institute of Process Engineering (IPE) and Dr. Jun Lu of Argonne National Laboratory in the United States discovered a “volcano-shaped” link between polysulfide adsorption and catalytic activity in lithium-sulfur (Li-S) batteries.
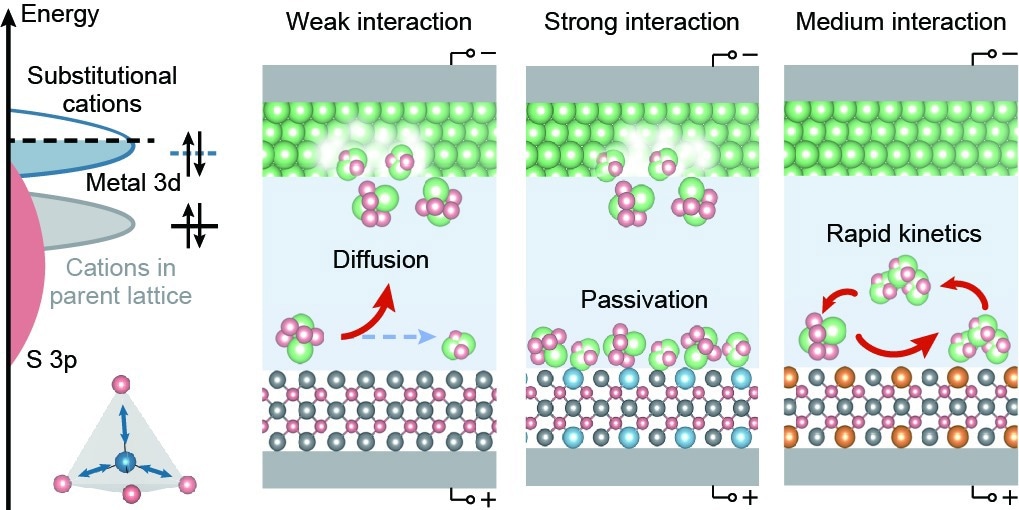 Schematic diagram of design principles for high-efficient lithium–sulfur catalyst. Image Credit: Zihan Shen
Schematic diagram of design principles for high-efficient lithium–sulfur catalyst. Image Credit: Zihan Shen
On June 16th, 2022, the study was published in Nature Catalysis.
This volcano-shaped connection, according to Prof. Zhang, might change the long-held premise that “high adsorption of polysulfides leads to excellent catalytic activity.”
Due to its high energy density, the Li-S system has a lot of potential for next-generation batteries. The slow kinetics of polysulfide conversion processes, on the other hand, causes the “shuttling effect,” which restricts rate capability and cyclability, making practical applications difficult.
Many recent experiments have found that catalytic polysulfide conversion is important for improving kinetics and inhibiting polysulfide shuttling. Despite substantial improvements in Li-S battery electrochemical performance, catalyst research has mostly depended on trial and error, with the guiding principle remaining elusive.
The researchers showed that despite polysulfide adsorption significantly lowering the activation barrier for polysulfide conversion, it also impedes product desorption in this study. This happens due to the scaling principle as polysulfides (from Li2S8 to Li2S2/Li2S) are successively adsorbed onto the same sites during charge/discharge.
They doped transition metal into the crystalline structure of ZnS to modulate adsorption energy and optimize catalytic efficiency. The dopants were strained, and their d-orbitals were adjusted correspondingly. As a result, the adsorption energy was linearly related to the dopant’s d-band center, while catalytic activity was “volcano-shaped.”
When desorption is rate-limiting, this study shows that a long-held concept of strengthening adsorption to improve catalysis is incorrect.
Catalysts and absorbents in a Li-S battery should be designed separately to improve the performance of Li-S batteries.
Huigang Zhang, Professor, Institute of Process Engineering, Chinese Academy of Sciences
This study provides a reasonable foundation for deciphering the catalytic mechanism of Li-S batteries at the atomic or molecular level, as well as engineering novel catalysts.
The National Key Research and Development Program of China, the National Natural Science Foundation of China, and the United States Department of Energy (DOE) all contributed to the project.
Journal Reference:
Shen, Z., et al. (2022) Cation-doped ZnS catalysts for polysulfide conversion in lithium–sulfur batteries. Nature Catalysis. doi:10.1038/s41929-022-00804-4.