Reviewed by Mila PereraSep 20 2022
Ammonia — a carbon-neutral energy carrier — is a prospective transportation fuel that has an extensive application in plastics, fertilizers, and explosives. Traditional ammonia synthesis approaches depend mainly on the high-pressure and high-temperature Haber–Bosch method, leading to significant greenhouse gas emissions and energy consumption.
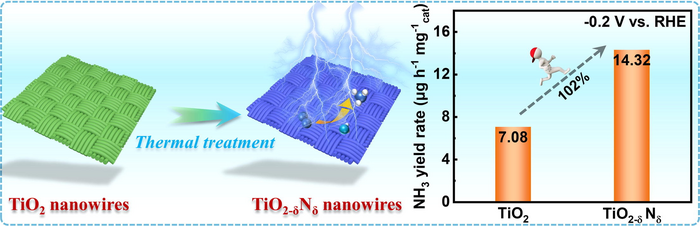 The catalysts based on TiO2−δNδ nanowires grown on carbon cloth feature a higher yield rate for ammonia than the electrocatalyst without N doping. Image Credit: Journal of Energy Chemistry
The catalysts based on TiO2−δNδ nanowires grown on carbon cloth feature a higher yield rate for ammonia than the electrocatalyst without N doping. Image Credit: Journal of Energy Chemistry
Developing the right solutions for attaining low-energy, high-efficiency, low-emission, and ecological ammonia production in benign environmental settings is crucial. The thermodynamically non-spontaneous ammonia synthesis reaction realized under ambient settings caused by electrical energy is enabled through electrochemical ammonia synthesis — a popular research topic.
Non-polar N2 is insoluble in water — which restricts its activation and adsorption on the catalyst surface. At the reduction potential, the competitive hydrogen evolution reaction considerably decreases the production and Faradaic efficiency of N2 reduction to ammonia. It is, therefore, important to identify novel electrocatalysts with high catalytic performance and examine the reaction mechanism of N2 reduction to ammonia.
Recently, in the Journal of Energy Chemistry, a paper was published by Professor Luo Wenbin at Northeastern University and Li Feng at the Insititute of Metal Research. Mu Jianjia, a doctoral student at Northeastern University, is the first author of this study. The co-author of this study is Gao Xuanwen, an Associate Professor. Professor Luo Wenbin and Li Feng are the corresponding authors.
To create an integrated network for evading binder-induced side reactions, 3D nanowire arrays were grown on carbon cloth in this research, increasing the cycling lifespan.
In 0.1 M KOH electrolyte, the nitrogen reduction reaction (NRR) showed a high ammonia production rate (14.33 µg h−1 mgcat−1) at -0.2 V and high Faradic efficiency (9.17%) at -0.1 V, outdoing the reported Ov-rich TiO2-based electrocatalysts.
In the NRR in ambient settings, the synergistic effects of Ov and Ti3+ were theoretically and experimentally demonstrated, thus showcasing a new concept for highly capable electrocatalysts.
Journal Reference
Mu, J., et al. (2022) Boosting nitrogen electrocatalytic fixation by three-dimensional TiO2−δNδ nanowire arrays. Journal of Energy Chemistry. doi.org/10.1016/j.jechem.2022.08.007.