Li-O2 batteries have advanced to become cutting-edge technology due to their high energy density. The production and decomposition of the discharged product solid lithium peroxide (Li2O2) inside the Li-O2 battery have a substantial impact on the battery’s performance.

Image Credit: Immersion Imagery/Shutterstock.com
Previous studies provided little insight into the form and distribution of internal Li2O2, leaving concerns over the pattern and causes of this shift in form and size unresolved.
A carbon-coated anodic aluminum oxide (C-AAO) air electrode with a highly-ordered, array-like structure was recently created by a team under the direction of Prof. Peng Tan from the University of Science and Technology of China (USTC).
The team learned new things about how and why Li-O2 batteries react and die suddenly. The research has been published in the journal Nano Letters.
The study team created a unique C-AAO electrode that breaks effortlessly but maintains its product distribution, allowing Li2O2 measurements across the entire electrode. The scientists identified the cause of the sudden voltage drop and mortality at different current densities using electrochemical impedance spectroscopy (EIS).
According to research, channel diameters limit the formation of toroidal Li2O2 at low currents, which results in electrode blockage.
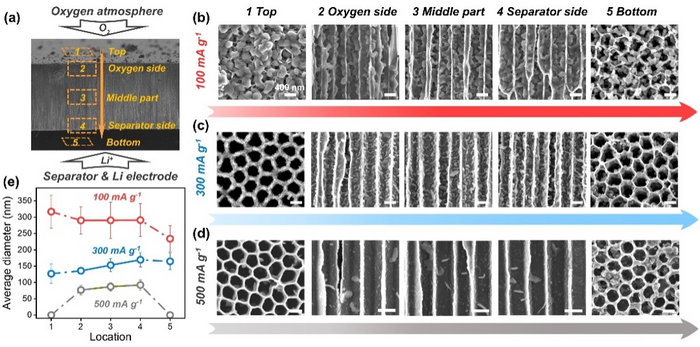
The distributions and sizes of lithium peroxide on the end surfaces of C-AAO electrodes and inside them. Image Credit: Prof. Peng Tan’s team.
Therefore, a high charge transfer impedance and concentration polarization brought on by electrode blockage are related to the abrupt voltage drop. While at high currents, the abrupt death is attributable to the concentration polarization and less significant charge transfer impedance from the quick electrochemical processes.
The research team also performed a thorough examination of the growth model of Li2O2 on the end surfaces and inside of C-AAO electrodes to determine the mechanism of such reactions.
Three toroidal models have Li2O2 on the end surfaces. The most typical one forms an incomplete ring by “hugging” the wall as it expands. The remaining material either forms nuclei on adjacent Li2O2 surfaces or expands laterally on the surface.
Toroidal Li2O2 inside the electrode is probably going to be covered by its flocculated counterparts as current density increases, showing that Li2O2 is created along the electrode’s surfaces rather than through disproportionation inside channels.
Lithium peroxide (LiO2) in solution disproportionate around Li2O2 particles, covering the surface route and forming an incomplete ring, after which Li2O2 generated at the Li2O2/electrode interface during early growth is related to the surface route. This new growth route was proposed by the team.
In addition to providing answers to long-standing queries about the Li-O2 battery’s machinations, this research also sheds light on future electrode design.
Journal Reference
Zhang, Z., et al. (2022) Reacquainting the Sudden-Death and Reaction Routes of Li–O2 Batteries by Ex Situ Observation of Li2O2 Distribution Inside a Highly Ordered Air Electrode. Nano Letters. doi:10.1021/acs.nanolett.2c02516.