A paper recently published in the journal Global Challenges reviewed the use of waste coffee grounds (WCGs) as electrode materials for high-performance electrode fabrication.

Study: Reusing Waste Coffee Grounds as Electrode Materials: Recent Advances and Future Opportunities. Image Credit: RasaBasa/Shuttertsock.com
Background
The upcycling of different waste and biomass into value-added functional materials is increasingly gaining attention to develop sustainable solutions and promote a circular economy. The coffee industry generates over eight million tons of WCGs every year. WCGs contain polyphenols, tannins, and caffeine, which can adversely affect the environment without proper disposal.
The macromolecular lignocellulose and cellulose present in WCGs can be used as inexpensive carbon precursors. Several carbon-based materials have been synthesized using WCGs, including particle-like graphene, graphene sheet fibers, carbon nanotubes, carbon nanosheets, mesoporous carbon, and activated carbon.
Electrodes synthesized using low-cost value-added WCG-derived carbon have demonstrated high chemical stability, polarization, and electrical conductivity. These electrodes have been used in electrochemical sensors, batteries, and supercapacitors/capacitors. In this study, the authors reviewed the recent advancements in electrodes synthesized using WCG-derived carbon.
Carbonization and Activation of WCGs
WCGs must be carbonized and activated to enable conductivity and improve resulting capacitance, respectively, before using them to fabricate high-performance electrodes.
Pyrolytic Carbonization
In this method, WCGs are pyrolyzed between 500 oC and 1000 oC in a tube furnace to synthesize electrode material for supercapacitors/capacitors and batteries. Typically, the WCGs are converted into activated carbon at 257–470 °C decomposition temperature. Subsequently, a high degree of graphitization and greater electrical conductivity are observed in the WCG-derived carbon when the temperature rises above 500 oC.
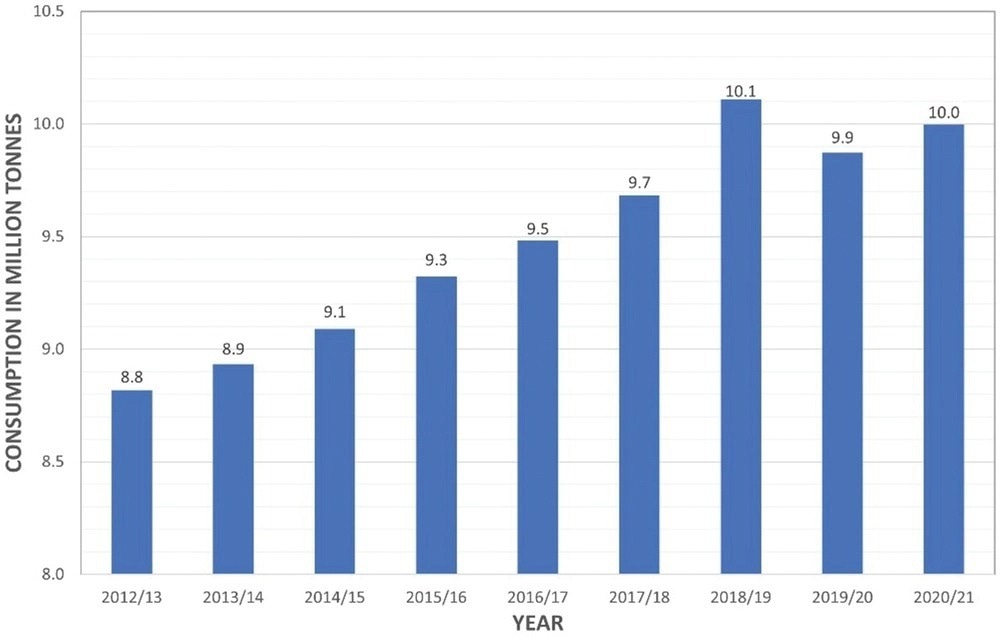
A graph to show the annual consumption of coffee worldwide in million tons. Image Credit: Pagett, M et al., Global Challenges
After pyrolysis, the resultant samples are activated using different methods, including chemical activation using different types of reagents and physical activation using carbon dioxide and steam, to increase the active surface area and porosity of the samples. These properties are commonly explored in WCG-derived carbon electrodes synthesized for electrochemical applications. The charge/discharge ability and the specific capacitance of the electrode increase corresponding with the increased surface area and porosity.
Microwave-assisted Carbonization
In 2015, a novel carbonization approach based on microwave plasma irradiation (MPI) was developed as a suitable alternative to conventional pyrolytic carbonization. In this carbonization method, MPI is used to produce different carbonaceous materials from WCGs. For instance, various types of long-wavy graphene-sheet fibers of different sizes were obtained from WCG using the MPI.
The WCG-derived graphene-sheet fibers demonstrated good capacitive behavior due to the unique morphology and greater specific surface area of graphene-sheet fibers, which indicated the feasibility of using WCG-derived graphene-sheet fibers for energy storage and electrochemical conversion applications.
Recent Applications of WCG-derived Carbon as Electrode Materials
WCGs were first successfully reused as electrode material in 2008 when nanoporous carbon electrodes synthesized using pyrolyzed coffee beans with zinc chloride activation were used as a low-cost alternative in supercapacitor applications.
Similarly, an asymmetrical energy storage device (AESD) was fabricated using WCG-derived carbon as a cathode for surface-driven storage of sodium ions. Core/shell microspheres with zinc oxide as core and WCG-derived carbon as shell were fabricated as an inexpensive electrode material for direct methanol fuel cells.
WCG-derived carbon as an electrode material for vanadium redox flow batteries (VRFBs) has demonstrated a higher voltage and energy efficiency compared to typical bipolar graphite during a static cell test. Moreover, WCG-derived carbon was used effectively as an anode material in sodium-ion batteries.
A hierarchically microporous activated carbon was fabricated successfully using WCGs to synthesize selenium (Se) cathodes for high-performance lithium (Li)-Se battery. Recently, WCG-derived biochar was utilized as a cost-efficient alternative for a conductive sulfur (S) host in a high-capacity Li/S battery.
Waste coffee biochar was used as low-cost carbon precursors after pyrolysis at 700 oC to fabricate humidity sensors. The sensor electrodes were synthesized using the screen-printing method. The fabricated electrodes displayed 20-100% impedance response for relative humidity and a short response and recovery time of less than two min.
Carbon paste obtained from WCGs was used to synthesize sensor electrodes to determine the heavy metal concentration in aqueous solutions. Sensor electrodes containing 50% WCG displayed the best results during differential pulse anodic stripping voltammetry determination of cadmium and lead ions at 89 × 10−6 M and 90 M limit of detection, respectively, indicating the potential of using WCGs in electrochemical sensor applications.
In 2021, carbonized WCGs were embedded into the silicone rubber elastomer as a shape-adaptive and lightweight capacitor for the first time to fabricate a novel, eco-friendly, and metal-free triboelectric nanogenerator (TENG) device. The fabricated wearable TENG device effectively harvested surrounding energy from human motions and stored this generated electricity in the WCG-derived capacitors to power portable electronics.
Moreover, the self-powered TENG also displayed the ability to sense human physiological signals, emulate gestures, and monitor motions. Thus, the device can be used to develop energy-efficient artificial sensors, smart tactile intelligent vending and epidermal controller coasters, and wearable electronics for human-machine interfaces and humanoid robotics.
Conclusion and Future Outlook
Recycling WCGs to other value-added functional materials is crucial to reduce waste generation by the beverage industry and promote a food circular economy. Recent studies have demonstrated the feasibility of using WCG-derived electrodes in different electrochemical applications. The use of WCGs as electrode material also leads to greater sustainability as it is a renewable source of carbon.
However, more research is required to assess the impact of impregnation ratios of different activation agents on the electrochemical performance and surface area of the WCG-derived electrodes, as pore size distribution is directly influenced by the impregnation ratio.
Additionally, the role of hydrophilic and hydrophobic functional groups, pore structure, and specific surface area in improving the electrochemical performance of WCG-derived carbon electrodes must be studied thoroughly.
In the future, novel carbonization methods, such as photonic annealing under an inert atmosphere and plasma jet-assisted carbonization, can be used and optimized to fabricate WCG-derived electrode materials.
Moreover, high-volume printing techniques, such as microfluidics, molecular imprinting, and nanofabrication techniques, can be used to realize large-scale production of cheap deposable electrochemical sensors using WCGs.
Reference
Pagett, M., Sullivan, G., Zhang, W. et al. Reusing Waste Coffee Grounds as Electrode Materials: Recent Advances and Future Opportunities. Global Challenges 2022. https://onlinelibrary.wiley.com/doi/10.1002/gch2.202200093
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.