Reviewed by Emily Henderson, B.Sc.Nov 15 2022
Researchers from the Chinese Academy of Sciences’ Hefei Institutes of Physical Science utilized controllably produced single-atom catalysts (SACs) to demonstrate the relationship between electrocatalytic nitrogen reduction reaction (NRR) performance and single-atom (SA) loading.
 Schematic diagram of ammonia synthesis by electrocatalysis with bimetallic Fe–Co single-atom catalyst. Image Credit: Shengbo Zhang.
Schematic diagram of ammonia synthesis by electrocatalysis with bimetallic Fe–Co single-atom catalyst. Image Credit: Shengbo Zhang.
The electrosynthesis of ammonia from NRR at ambient conditions has been generally touted as a “green ammonia synthesis” method that could replace the existing energy- and capital-intensive Haber-Bosch process.
Experts agree that the remarkable properties of SACs may lead to the development of a new catalytic paradigm. However, one of the major impediments to rational SAC design and development is a lack of understanding of the relationship between performance and SA loading, owing to the inability to accurately control the synthesis of SACs with appropriate SA loading densities and active site coordination forms.
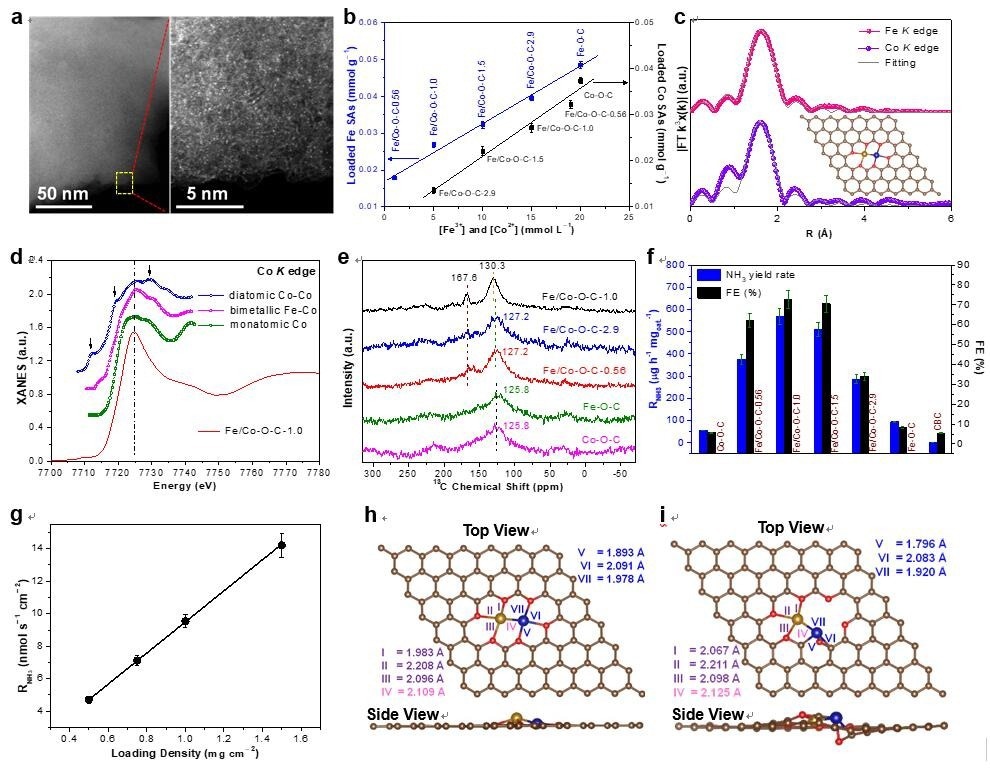
The investigators presented an adsorption-regulated synthetic technique that employs bacterial cellulose as an adsorption regulator to regulate Fe3+/Co2+ impregnation on bacterial cellulose through carbothermal reduction. Bimetallic [(O–C2)3Fe–Co(O–C2)3] coordination was then used to attach Fe-Co SAs to bacterial cellulose-derived carbon.
Notably, the researchers revealed a set of quantitative relationships that classify the distribution of Fe3+/Co2+ between bacterial cellulose and the adsorption solution, as well as the percentage conversion of impregnate Fe3+/Co2+ on bacterial cellulose to Fe/Co SAs on bacterial cellulose-derived carbon.
Scientists subsequently showed how such quantitative connections could direct the controllable synthesis of bimetallic Fe-Co SACs with predefined Fe/Co contents and atomic ratios.
Researchers demonstrated that SACs produced in a controlled manner could display the electrocatalytic link between NRR performance and SA loading. Single-atom electrocatalysts (SAECs) with a unity Fe/Co atomic ratio have the maximum site density and NRR performance for bimetallic Fe-Co SAs, allowing them to achieve a high ammonia production rate while maintaining excellent faradaic efficacy.
Unlike other types of catalysts, the catalytic activity of SACs is affected by the nature of the SA, the physiochemical properties of the support, and, most crucially, the coordination bonds that attach the SA to the support.
Under electrocatalytic NRR conditions, [(O-C2)3Fe-Co(O-C2)3] in the as-synthesized bimetallic Fe-Co SAECs is operando transformed into the highly stable coordination configuration [(O-C2)3Fe-Co(O-C)C2], thus enhancing and prolonging NRR performance.
According to the researchers, the recent results will be of tremendous interest to the entire catalysis community.
Journal Reference:
Zhang, S. et al. (2022) Atomically dispersed bimetallic Fe–Co electrocatalysts for green production of ammonia. Nature Sustainability. doi.org/10.1038/s41893-022-00993-7.