Electric cars are often viewed as the best option for replacing traditional cars with a more environmentally friendly alternative. However, electric cars and other electric vehicles will almost certainly be powered by lithium-ion batteries, which currently do not provide the required performance and durability at an affordable price.
 Scientists have produced a new high-capacity electrode material that barely changes in volume during the charge/discharge process. This unique feature can be leveraged to produce solid-state lithium-ion batteries with unprecedented durability. Image Credit: Yokohama National University.
Scientists have produced a new high-capacity electrode material that barely changes in volume during the charge/discharge process. This unique feature can be leveraged to produce solid-state lithium-ion batteries with unprecedented durability. Image Credit: Yokohama National University.
Solid-state batteries (SSBs), on the other hand, have gained popularity among academics seeking alternatives in recent years. Unlike traditional lithium-ion batteries, which have a liquid electrolyte through which lithium ions move during the charge/discharge process, SSBs are entirely composed of solid materials.
Aside from a significant increase in operational safety (since these batteries will not discharge toxic liquids when punctured), SSBs can be charged significantly faster.
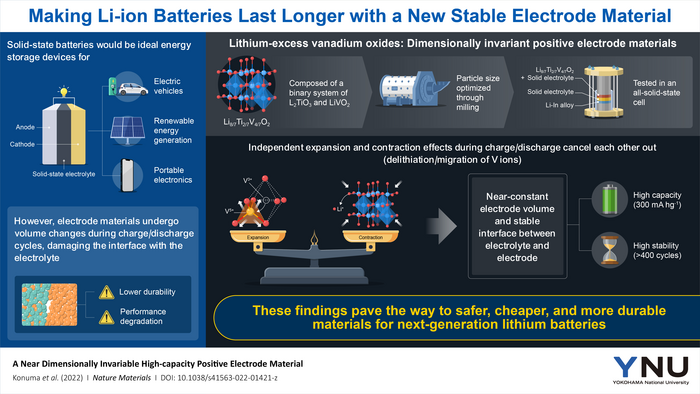
However, an unresolved issue in SSBs restricted their durability until recently. When lithium ions are introduced into or withdrawn from the battery’s electrodes, the crystalline structure of the material changes, causing the electrode to expand or contract; these repetitive fluctuations in volume harm the contact between the electrodes and the solid electrolyte and create irreversible abnormalities in the crystal chemistry of the electrodes.
A team of scientists led by Professor Naoaki Yabuuchi of Yokohama National University in Japan explored a new type of positive electrode material with remarkable stability in SSBs against this backdrop. Associate Professor Neeraj Sharma of UNSW Sydney, Australia, and Dr. Takuhiro Miyuki of LIBTEC, Japan, collaborated on the study, which was published in Nature Materials.
The research team concentrated on Li8/7Ti2/7V4/7O2, a binary system made up of optimum amounts of lithium titanate (Li2TiO3) and lithium vanadium dioxide (LiVO2). When ball-milled to an acceptable particle size in the nanoscale range, this material provides high capacity due to the enormous quantity of lithium ions that can be entered and withdrawn reversibly during the charge/discharge process.
Li8/7Ti2/7V4/7O2 has a unique attribute that distinguishes it from other positive electrode materials: it has approximately the same volume when fully charged and discharged. The researchers investigated the source of this trait and came to the conclusion that it is the consequence of a delicate balance between two distinct processes that take place when lithium ions are introduced or withdrawn from the crystal.
On the one hand, the elimination of lithium ions, known as “delithiation,” increases the free volume of the crystal, causing it to shrink. Some vanadium ions, on the other hand, move from their initial position to the vacancies left behind by the lithium ions, gaining a higher oxidation state in the process. This results in a repulsive interaction with oxygen, which causes the crystal lattice to expand.
When shrinkage and expansion are well balanced, dimensional stability is retained while the battery is charged or discharged, i.e. during cycling. We anticipate that a truly dimensionally invariable material – one that retains its volume upon electrochemical cycling – could be developed by further optimizing the chemical composition of the electrolyte.
Naoaki Yabuuchi, Professor, Yokohama National University
This new positive electrode material was tested in an all-solid-state cell by mixing it with a suitable solid electrolyte and a negative electrode. This cell's capacity was 300 mA.h/g with no degradation after 400 charge/discharge cycles.
The absence of capacity fading over 400 cycles clearly indicates the superior performance of this material compared with those reported for conventional all-solid-state cells with layered materials. This finding could drastically reduce battery costs. The development of practical high-performance solid-state batteries can also lead to the development of advanced electric vehicles.
Neeraj Sharma, Associate Professor, UNSW Sydney
It may soon be possible to make batteries suitable for electric vehicles in terms of price, capacity, safety, charging speed, and lifespan by improving dimensionally invariant electrode materials.
The development of long-life and high-performance solid-state batteries would solve some of the problems of electric vehicles. In the future, for instance, it may be possible to fully charge an electric vehicle in as little as five minutes.
Naoaki Yabuuchi, Professor, Yokohama National University
Scientists expect to see more advancement in this area, which will assist in expediting the adoption of electric vehicles and contribute to a better future for the globe.
Journal Reference:
Konuma, I., et al. (2022) A near dimensionally invariable high-capacity positive electrode material. Nature Materials. doi.org/10.1038/s41563-022-01421-z.