Electrocatalysis is important in many clean energy conversion technologies, and it can be combined with renewable energy generation systems like photovoltaics, wind turbines, and hydropower to tackle future global energy and climate issues.
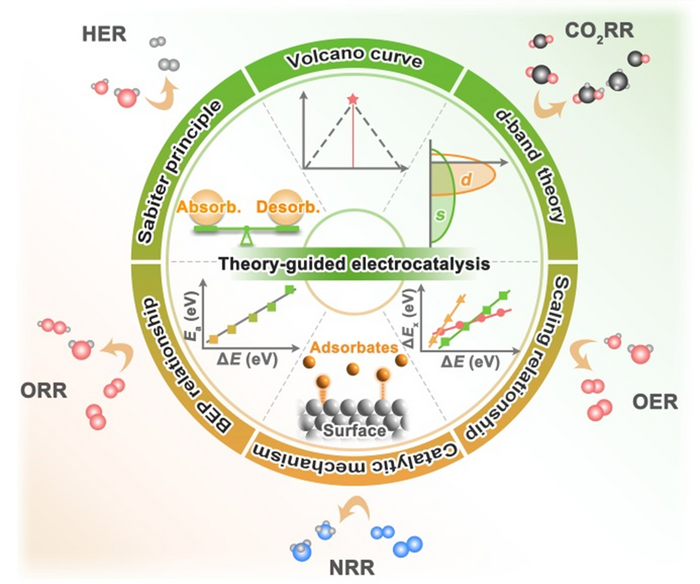
The rational design of catalysts needs to be based on theoretical guidance. We summarize the development and content of classical theories in the field of electrocatalysis. Including Sabiter principle, Volcano curve, Brønsted-Evans-Polanyi relationship, Scaling relationship, and descriptors commonly used in a series of electrochemical reactions (HER, OER, ORR, NRR and CO2RR). Image Credit: Chinese Journal of Catalysis.
Hydrogen evolution reactions (HER), oxygen evolution reactions (OER), oxygen reduction reactions (ORR), nitrogen reduction reactions (NRR), and carbon dioxide reduction reactions (CO2RR) are some major electrochemical conversion mechanisms that have piqued the curiosity of researchers.
Under benign conditions, these electrochemical reactions are capable of converting plentiful natural resources into significant compounds (hydrogen, ammonia, and organics). However, because these electrochemical reactions are kinetically slow, high-performance electrocatalysts are required to boost reaction effectiveness and decrease extra energy consumption.
Current electrocatalysts are deficient in activity, stability, and selectivity and are heavily reliant on noble metals. The development of novel electrocatalysts is vital.
Conventional electrocatalyst research and development is based on trial-and-error methods, which are time-consuming and costly. Over the last two decades, the creation of new materials based on theoretical guidance has evolved into more sophisticated design ideas for electrocatalysts, owing primarily to the emergence of important basic theories, activity descriptors, and catalyst mechanisms, as well as the maturity of computational chemistry in the field of electrochemistry.
These breakthroughs show the structural-activity law of electrocatalysts and speed up the electrocatalyst development process.
A team from Jilin University, China, headed by Prof. Xiaoxin Zou and Prof. Hui Chen, recently explored the development process of electrocatalyst guiding theory. Starting with the early qualitative Sabiter principle, Volcano curves, Brnsted-Evans-Polanyi relations, and scaling relations have become key theories for quantitatively describing catalyst activity with the use of computational chemistry.
The theory of the d-band center and the d-band regulation technique (strain effect and ligand effect) are thoroughly presented.
By merging the most recent high-throughput screening and machine learning approaches, the mainstream reaction mechanisms, theoretical research advances, and descriptors for important electrocatalytic reactions (HER, OER, ORR, NRR, and CO2RR) were summarized. The challenges and prospects for developing high-performance electrocatalysts based on theory are also highlighted.
Journal Reference:
Zhang, M., et al. (2022) Theory-guided electrocatalyst engineering: From mechanism analysis to structural design. Chinese Journal of Catalysis. doi.org/10.1016/S1872-2067(22)64103-2.