Electrochemical devices, such as fuel cells, are becoming indispensable for new power production technologies due to their ability to efficiently produce renewable energy. Numerous devices, such as hydrogen pumps, sensors, separation membranes, and protonic ceramic fuel cells (PCFCs), can make use of ceramic proton conductors.
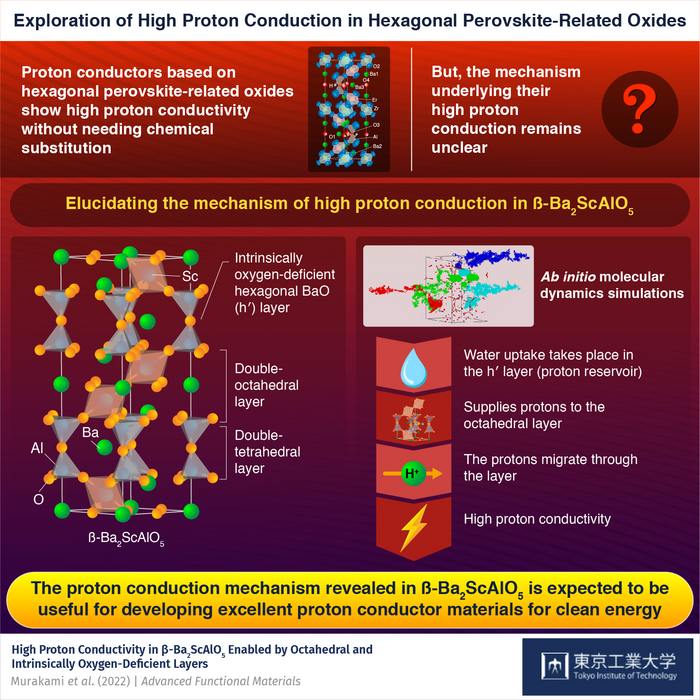
Image Credit: Tokyo Tech
In particular, the PCFCs based on ceramic proton conductors are promising because they can work at lower temperatures compared with the conventional solid oxide fuel cells (SOFCs), thanks to the higher conductivity of protons at low temperatures.
The issue with conventional ceramic proton conductors is that they require oxygen vacancies that allow for the incorporation of water to exhibit adequate proton conductivity. The vacancies are typically produced by chemical substitution, which is frequently a challenging process.
Instead, a team of researchers under the direction of Professor Yashima of the Department of Chemistry at Tokyo Tech have studied the proton-conducting oxides associated with hexagonal perovskites.
The crystal structure of these oxides includes layers that are inherently oxygen-deficient, which results in high proton conductivity without chemical replacement. However, their conduction mechanism remains unclear.
Professor Yashima’s research team recently compared and analyzed three different types of oxides, including β-Ba2ScAlO5, α-Ba2Sc0.83Al1.17O5, and BaAl2O4, to shed light on this. All three of these oxides have different stacking patterns for their oxide-deficient layers.
The researchers discovered that while β-Ba2ScAlO5 had a high proton conductivity, α-Ba2Sc0.83Al1.17O5 and BaAl2O4, which were structurally related, had much lower conductivities. Advanced Functional Materials published the team's findings.
It consists of double-octahedral layers separated by double-tetrahedral layers. The latter have hexagonal BaO (h’) layers that are intrinsically oxygen deficient. Their roles in proton conduction have been explored through various methods.
Dr. Masatomo Yashima, Professor, Department of Chemistry, Tokyo Institute of Technology
First, the scientists discovered that the ion conductivity of β-Ba2ScAlO5 was significantly (31 times) higher in wet conditions than in dry air. This was caused by the material absorbing water from the humid air, which increased conductivity and proton concentrations.
Above 300 °C, it was discovered that the proton conductivity reached as high as 10–3 S cm–1, a level that is comparable to that of conventional, chemically substituted conductors.
Calculations using bond valence-based energy and density functional theory showed that this water uptake takes place in the oxide’s h′ layers. Furthermore, ab initio molecular dynamics simulations demonstrated that these layers serve as reservoirs, supplying protons that migrate in the double-octahedral layers via long-range diffusion. This phenomenon causes β-Ba2ScAlO5 to have a high proton conductivity.
BaAl2O4, in contrast, showed significantly lower conduction because it absorbed less water, had low proton mobility, and lacked octahedral layers. These findings further validate the significant roles of both octahedral and oxygen-deficient layers in proton conduction.
The study is a great example of tackling complex research problems through collaboration and showcases ANSTO capabilities and expertise in neutron scattering and scientific computing. The Echidna diffractometer at the OPAL reactor was used to elucidate crystal structure and molecular dynamics simulations also performed at ANSTO shed light on the proton conductivity mechanism.
Max Avdeev, Professor, Neutron Diffraction Group Manager, Australian Centre for Neutron Scattering, Australian Nuclear Science and Technology Organization
While pointing out the future potential of the team’s work, Prof. Yashima stated, “Our results offer a strategy for designing superior hexagonal perovskite-related oxides with octahedral layers and intrinsically oxygen-deficient layers. Combining these layers with different roles can produce superior proton conductors for renewable energy production and storage devices.”
Journal Reference:
Murakami, T., et al. (2022) High Proton Conductivity in β-Ba2ScAlO5 Enabled by Octahedral and Intrinsically Oxygen-Deficient Layers. Advanced Functional Materials. doi:10.1002/adfm.202206777.