A chemistry partnership resulted in a novel approach to deploying carbon dioxide to good—and even healthy—use: by electrosynthesizing it into a series of organic molecules critical to pharmaceutical research.
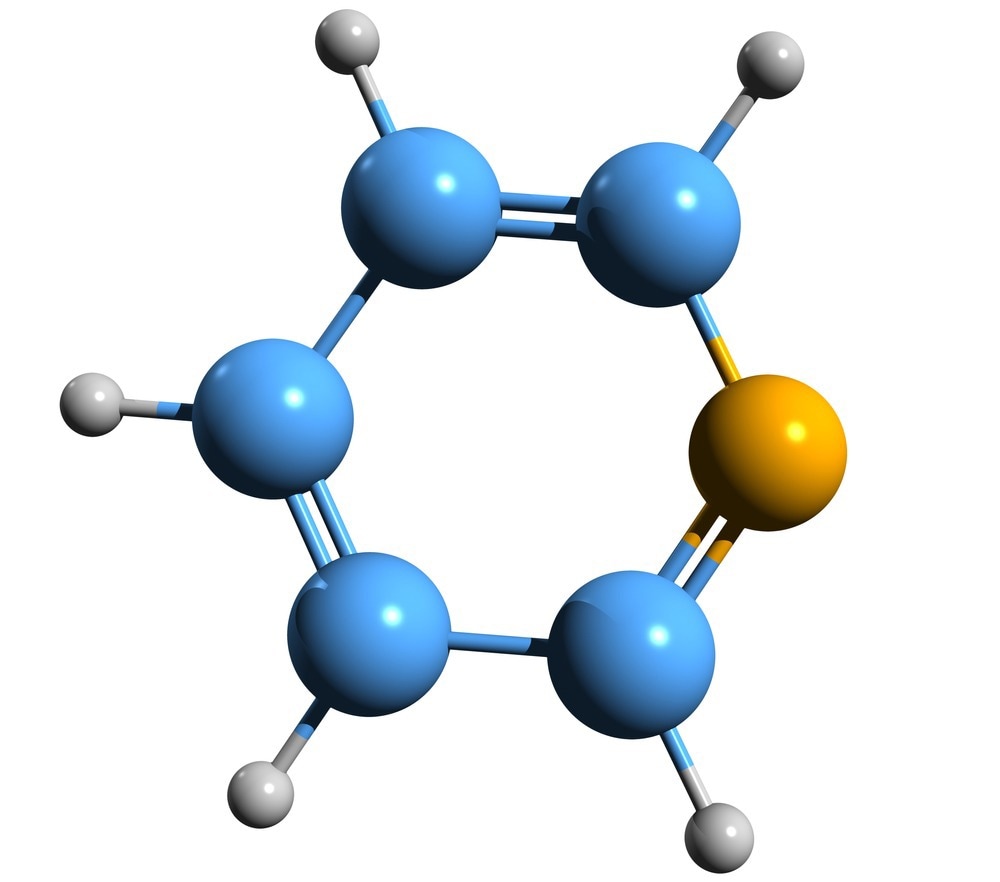
Image Credit: Zita/Shutterstock.com
During the approach, the team made a novel finding. They could make two completely distinct products, both of which are valuable in medicinal chemistry, by altering the type of electrochemical reactor.
The study was published in the journal Nature on January 5th, 2023. Postdoctoral researchers Peng Yu and Wen Zhang, as well as Guo-Quan Sun of Sichuan University in China, are the paper’s co-lead authors.
The Cornell group, headed by Song Lin, a Professor of chemistry and chemical biology in the College of Arts and Sciences, has earlier combined simple carbon molecules to create complex compounds using the electrochemical process, doing away with the need for precious metals or other catalysts to speed up the chemical reaction.
Researchers narrowed their focus for the new initiative to pyridine, the second-most common heterocycle among FDA-approved drugs. Heterocycles are organic compounds in which the atoms of the molecules are connected into ring structures, one of which is not carbon. These structural units are known as “pharmacophores” because they are frequently found in medicinally active substances. They are also widely found in agrochemicals.
The researchers wanted to create carboxylated pyridines, which are pyridines with carbon dioxide attached to them. The addition of carbon dioxide to a pyridine ring has the advantage of changing the functioning of the molecule and ultimately assisting it in binding to certain targets like proteins. The two molecules, however, are not natural companions. Pyridine is a reactive molecule, whereas carbon dioxide is an inert gas.
“There are very few ways of directly introducing carbon dioxide to a pyridine. The current methods have very severe limitations,” added said Lin, the co-senior author of the paper, along with Da-Gang Yu of Sichuan University.
Lin's laboratory successfully synthesized carboxylated pyridines by combining its electrochemistry skills with Yu’s group’s experience in using carbon dioxide in organic synthesis.
Electrochemistry gives you that leverage to dial in the potential that is sufficient to activate even some of the most inert molecules. That’s how we were able to achieve this reaction.
Song Lin, Professor, Chemistry and Chemical Biology, College of Arts and Sciences, Cornell University
While performing the electrosynthesis, the researchers made a coincidental finding. An electrochemical reaction is normally carried out in one of two ways by chemists: either in an undivided electrochemical cell (where the anode and cathode that supply the electric current are in the same solution) or in a divided electrochemical cell (where the cathode and anode are separated by a porous divider that blocks huge organic molecules but allows ions to pass through). Although one strategy is more efficient than the other, they both generate the same product.
Lin's group discovered that transitioning from a divided to an undivided cell allowed them to selectively attach the carbon dioxide molecule to different places of the pyridine ring, resulting in two distinct products: C4-carboxylation in the undivided cell and C5-carboxylation in the divided cell.
This is the first time we discovered that by just simply changing the cell, what we call the electrochemical reactor, you completely change the product. I think that mechanistic understanding of why it happened will allow us to continue to apply the same strategy to other molecules, not just pyridines, and maybe make other molecules in this selective but controlled fashion. I think that’s a general principle that can be generalized to other systems.
Song Lin, Professor, Chemistry and Chemical Biology, College of Arts and Sciences, Cornell University
While the project's method of utilizing carbon dioxide will not solve the world-wide problem of climate change, Lin stated, “it’s a small step towards using excessive carbon dioxide in a useful way.”
The study co-authors included postdoctoral researcher Yi Wang and doctoral student Zhipeng Lu; and researchers from Sichuan University.
National Institute of General Medical Sciences, Eli Lilly, Cornell, and the Sloan Foundation funded the research.
Journal Reference
Sun, G.-Q., et al. (2023) Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations. Nature. doi.org/10.1038/s41586-022-05667-0.