Rapid results testing has become important in today’s fast-paced world. Droplet microfluidics enable faster point-of-care testing (POCT), although it is not necessarily as precise and necessitates some additional effort for optimal handling.
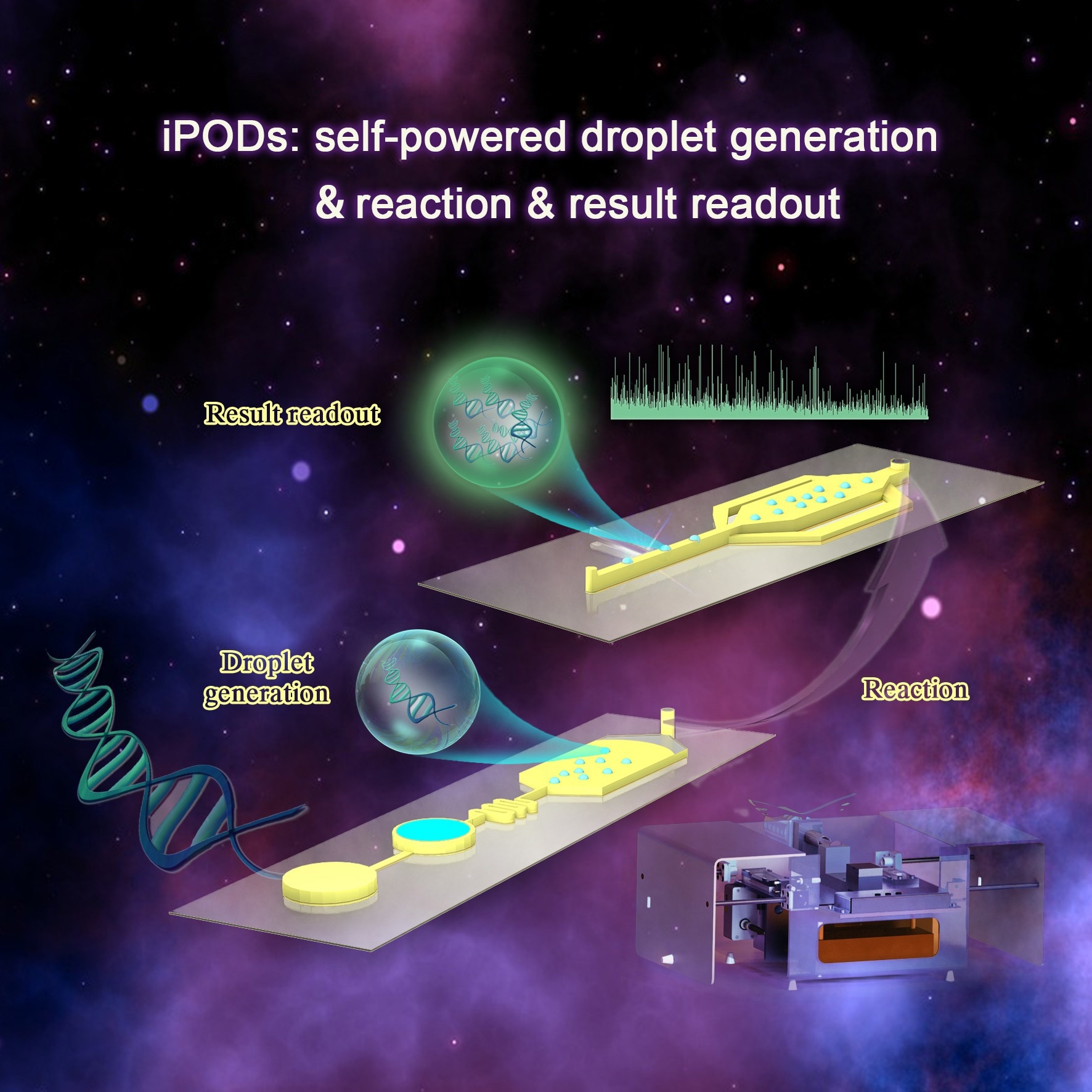
iPODs for portable integrated droplet applications. Image Credit: Yang Liu
The Integrated Portable Droplets (iPODs) system, created by researchers from the Single-Cell Center at Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS), is based on an auto flow-focusing droplet reinjection chip that eradicates the need for external pumps and accurate fluid control, enabling for portability, lower costs, and a user-friendly method of droplet analysis.
On April 13th, 2023, the study was published in Analytical Chemistry.
The scientists found positive results: the automated method in which the droplets pass through the instrument eliminates the need for manual operation, lowering the risk of error, cross-contamination, and sample loss.
They also discovered that samples measured between 101 and 104 copies of bacteria per microliter had a significant linearity with an R2 value of 0.999. The greater the R2 value, the better the line can visually reflect how well the iPODs device (the independent variable) can identify and measure the bacterial species present in the sample (the dependent variable).
Furthermore, the device cost and the following cost of each use are reasonable enough to be widely affordable. Another distinct and important feature of the device is its portability, which allows it to be used in a variety of contexts, such as outdoor settings or decentralized labs.
In previous reports, droplet reinjection undoubtedly requires precise fluid control, thereby reducing the portability of the device, more seriously, restricting the development of droplet-based nucleic acid amplification testing for POCT. Here we showed this droplet reinjection chip greatly simplified the experimental setup and operation procedure with low device cost and low reagent consumption.
Fengyi Liu, Study First Author and Doctoral Student, Single-Cell Center, Chinese Academy of Sciences
Droplet microfluidic testing entails analyzing a small amount of fluid, which frequently includes cell manipulation and single-cell or single-molecule analysis. One difficulty addressed by iPODs is the comparatively low quantitative detection levels of loop-mediated isothermal amplification (LAMP) when performed in bulk, which results in an “all or nothing” result.
Droplet digital LAMP using iPODs is highly sensitive and accurate in determining the number of nucleic acids contained in a sample. The disadvantage is that precise fluid management and external pumps are required, which reduces portability and increases costs.
“We present a droplet reinjection method capable of droplet distribution without precise fluid control and external pumps, by which the droplets can be passively aligned and detected one by one at intervals,” said Anle Ge, contributing author and assistant research fellow.
The integration of the droplet generator, heating tool, and fluorescent signal reader, all bundled in a small, retractable box, eliminates the need for an additional pump and precise fluid control when using iPODs.
Some improvements will be required in the future for this technology to realize its full potential, such as enhancing system stability and implanting thermal cycling modules for droplet digital PCR (ddPCR). The addition of alternative low-cost chip materials for large-scale standardized manufacture, as well as the implementation of completely automated operation processes, will make the system more user-friendly.
Once the device is fully developed, we hope to see the technology used in a wide array of applications, from biochemical testing at a point-of-care level to in a more clinical research-based setting.
Bo Ma, Study Corresponding Author and Professor, Single-Cell Center, Chinese Academy of Sciences
Journal Reference
Liu, F., et al. (2023). Auto Flow-Focusing Droplet Reinjection Chip-Based Integrated Portable Droplet System (iPODs). Analytical Chemistry. doi.org/10.1021/acs.analchem.3c00239.